Translational Perioperative and Pain Medicine (ISSN: 2330-4871)
ARTICLE DOI: 10.31480/2330-4871/136
Review Article Open Access
Medical and Surgical Treatment of Atrial Fibrillation
Eric B. Brown, MD1, George J. Arnaoutakis, MD2 and Yong G. Peng, MD, PhD, FASE, FASA1*
1Department of Anesthesiology, University of Florida College of Medicine, Gainesville, FL, USA
2Division of Thoracic and Cardiovascular Surgery, University of Florida College of Medicine, Gainesville, FL, USA
Yong G. Peng, MD, PhD, FASE, FASA, Department of Anesthesiology, University of Florida College of Medicine, 1600 SW Archer Road, Gainesville, FL, 32610, USA, Tel: +1-352-294-3727, Fax: 352-392-7029, E-mail: ypeng@anest.ufl.edu
Editor: Renyu Liu, MD, PhD, Professor, Department of Anesthesiology and Critical Care, Perelman School of Medicine at the University of Pennsylvania, Center of Penn Global Health Scholar, Director of Stroke 120 Special Task Force, Chinese Stroke Association, 336 John Morgan Building, 3620 Hamilton Walk, Philadelphia, PA 19104, USA, Phone: 2157461485, Fax: 2153495078, E-mail: RenYu.Liu@pennmedicine.upenn.edu
Received: March 05, 2021 | Accepted: March 09, 2021 | Published: March 10, 2021
Citation: Brown EB, Arnaoutakis GJ, Peng YG. Medical and Surgical Treatment of Atrial Fibrillation. Transl Perioper & Pain Med 2021; 8(2):329-336
Abstract
As the world's growing population ages, diseases associated with the elderly, such as atrial fibrillation (AF), have increased in prevalence. These patients require specialized anesthetic care when presenting for surgical procedures, as they often have additional cardiovascular comorbidities and are frequently anticoagulated. Innovative techniques promise to mitigate thromboembolic risks associated with AF or even provide a cure. However, many are highly technical and associated with unique and potentially serious complications. Increasingly, comfort with providing anesthetics in nontraditional locations and use of advanced transesophageal echocardiography are required of anesthesiologists to safely and competently provide care during these novel procedures. The majority of left atrial appendage occlusion devices are percutaneously delivered and require intensive fluoroscopy or transesophageal echocardiography both for device placement and evaluation of possible side effects, such as leaking around seal devices, pericardial effusion, or tamponade. Catheter-based ablations that seek to both map and eliminate arrhythmogenic foci via different energy sources are associated with numerous side effects, including cardiac tamponade, hemo- and/or pneumothorax, vascular access-related complications, stroke, phrenic nerve injury, pulmonary vein stenosis, atrioesophageal fistula, and death. Due to its proximity to the pulmonary veins, mild esophageal ulceration occurs frequently with energy delivery. Patients who develop atrioesophageal fistula often present in extreme circumstances and require a multidisciplinary approach on an emergent basis before operative management. The modern anesthesiologists, therefore, must be familiar with these procedures to care for these patients and mitigate damage from unintended side effects.
Keywords
Anticoagulation, Atrial fibrillation, Cardiac surgery, Catheter ablation, Left atrial appendage
Introduction
As the world's population has aged, a larger number of sicker patients with comorbidities are seeking care, requiring a multidisciplinary health-care approach. This care more frequently comes in the form of innovative interventional procedures that are less invasive and therefore thought to be more suitable for high-risk or traditionally inoperable surgical patients. As they require newer, more specialized equipment, these novel procedures are increasingly being performed in ambulatory surgical centers and cardiac catheterization, endoscopy, and radiological suites, and less often in operating rooms. An increasing percentage of proceduralists are not surgeons and non-operating room nursing and support staff are often employed, adding another layer of separation from conventional practice. In most clinical scenarios, anesthesia personnel are still required to be present to ensure the safety of sedation, immediate resuscitation for unexpected events, and overall patient well-being. Anesthesia providers are expected to provide comprehensive services for complex or critically ill patients in these locations, all within the constraints of normal working hours.
Atrial fibrillation (AF) is a common medical condition in the geriatric population and presents unique challenges [1]. As the world's leading cause of atrial arrhythmia, this disease affects an increasing percentage of the population and places this group of patients at an increased risk of periprocedural complications, mainly related to thromboembolic phenomena. In addition to presenting with significant comorbidities, this subset of patients uses unique medication therapies that can complicate anesthetic planning for surgery. Novel procedures for the management and treatment of AF require specialized care and can provide a cure in some cases. However, rare but serious complications are being reported as the number of procedures has increased over the past decade. Knowledge of these sequelae is essential and can prove life-saving as many of these patients will re-present in extreme circumstances.
Medical Treatment of AF and Conflict with Surgery
A disease that primarily affects the elderly, AF is very rare below the age of 50 but the incidence increases to 10% at the age of 80 [2]. Risk factors include alcohol use, obstructive sleep apnea, obesity, diabetes mellitus, hypertension, cardiac valvular diseases, and coronary artery disease. These comorbidities decrease a patient's overall quality of life and, unfortunately, decrease the chances of successful treatments for an arrhythmia [3]. The disorganized activity of AF is thought to originate as premature atrial contractions from ostia of the pulmonary veins as they enter the left atrium. Over time, fibrotic tissue changes around conduction systems lower the success rate of effective treatments and allow the disease to persist [4]. Medical therapies attempt to control either heart rate or rhythm, but these therapies largely aim to treat symptoms and improve quality of life rather than provide a cure [5].
Stasis of blood flow in the left atrium and left atrial appendage (LAA) has a tendency to form thrombus; additional medical therapy is essential to prevent the formation of clots and lower the risk of stroke from thromboembolic events. Systemic anticoagulation is recommended when the CHA2DS2-VASC score is greater than 2 [6]; in the outpatient setting, this is most often accomplished with oral medications. Anticoagulants put patients at risk of gastrointestinal and cerebral bleeds; guidelines exist that aim to appropriately stratify patients and prevent unnecessary risks [7]. Patients on anticoagulants requiring emergency or urgent procedures are at increased risk for bleeding complications. Anesthesiologists can be limited in their ability to perform regional and neuraxial procedures, altering anesthetic planning and potentially exposing the patient to more risk. The timing to cease these therapies requires advance planning because the newer classes of anticoagulant medicines do not have readily available antidotes; 12 hours or more lagging time without specific therapy are required to reverse their effects [8]. This withholding period before surgery may put patients at risk for intracardiac thrombus formation.
Therapeutic Devices for Treatment of AF
In patients with an excessive bleeding risk or intolerance to the side effects or dosing schedules of anticoagulants, a variety of methods to exclude the LAA can be employed. A surgical approach, via open sternotomy or thoracotomy (or, more recently, thoracoscopy) is often applied in combination with other cardiac procedures (e.g., valve replacements/repairs) when concomitant AF is present. These involve the excision or suturing of the LAA. A novel device, using the AtriClip LAA Exclusion System (AtriCure, Mason, OH, USA), accomplishes the same goal more efficiently using an external closure device [9]. A similar device, the TigerPaw System (Terumo Cardiovascular Systems, Ann Arbor, MI), is no longer available. These procedures are performed under general anesthesia and, while transesophageal echocardiography (TEE) may be performed during the operation, echo guidance is not necessary during the LAA occlusion portion because this relies more on direct visualization and surgical technique for successful LAA cavity obliteration. In addition to the morbidity associated with undergoing open heart surgery as well as longer operating room times, retrospective analyses have found these techniques to be limited by a high incidence of incomplete closure of the LAA, subjecting these patients to the original adverse risk of thromboembolic events [10,11]. This is the subject of ongoing study in surgical populations.
Similarly, the involvement of anesthetic personnel is expected for percutaneous, catheter-based techniques, which are more commonly taking place in non-operating room settings, such as cardiac catheterization laboratories, electrophysiology laboratories, or hybrid operating rooms, and may or may not involve the presence of cardiothoracic surgeons (Table 1). Currently, only one device is approved by the U.S. Food and Drug Administration (FDA) for use in humans. This device, the Watchman LAA Occluder (Boston Scientific, Marlborough, MA), involves the endovascular delivery of an intracardiac device via a transseptal approach. Another device, the LARIAT system (SentreHEART Inc., Redwood City, CA), has CE Mark status and involves a hybrid approach where the appendage is ligated epicardially with guidance from removable endovascular, intracardiac support devices. Other devices without FDA approval include the WaveCrest (Biosense Webster, Irvine, CA), the Occlutech (Occlutech, Helsingborg, Sweden), and the LAmbre (Lifetech Scientific Corp., Shenzhen, China). The Amplatzer Cardiac Plug (St. Jude Medical, St. Paul, MN) is approved only for atrial septal defect closure but not occlusion of the LAA; however, testing of the newer Amulet device has begun. Similar to the LARIAT device, the Sierra Ligation System (Aegis Medical Innovations, Vancouver, Canada) is completely epicardially delivered and FDA-approved human trials have recently begun. As each device has specific inclusion and exclusion criteria, and the anatomy of the LAA is highly variable, it is advantageous to have a variety of mechanisms with which to occlude the LAA.
Table 1: Variable medical devices applied for atrial appendage closure.
|
Device |
Approach |
Requires Transesophageal Echocardiography |
Intra-cardiac Material |
FDA Approval Status |
|
AtriClip LAA Exclusion System (AtriCure, Mason, OH) |
Surgical, open vs thoracoscopic |
No |
No |
Approved |
|
Watchman LAA Occluder (Boston Scientific, Marlborough, MA) |
Endovascular via transseptal approach |
Yes |
Yes |
Approved |
|
LARIAT (SentreHEART, Redwood City, CA) |
Hybrid (endovascular + percutaneous epicardial) |
No |
No |
CE Mark |
|
WaveCrest (BioSense Webster, Irvine, CA) |
Endovascular via transseptal approach |
Yes |
Yes |
No |
|
Amulet (St. Jude Medical, St. Paul, MN) |
Endovascular via transseptal approach |
No |
Yes |
CE Mark |
|
Occlutech (Occlutech, Helsingborg, Sweden) |
Endovascular via transseptal approach |
No |
Yes |
No |
|
LAmbre (Lifetech Scientific, Shenzhen, China) |
Endovascular via transseptal approach |
No |
Yes |
No |
|
Ultraseal LAA Device (Cardia Inc., Eagan, MN) |
Endovascular via transseptal approach |
No |
Yes |
No |
|
Sierra Ligation System (Aegis Medical Innovations, Vancouver, Canada) |
Percutaneous epicardial |
Yes |
No |
No |
Providers should pay special attention to renal function and anticoagulation status through patient history and physical exams prior to performing these procedures, as well as ensure there is no clot within the LAA via perioperative TEE. Continuous TEE guidance is required throughout the deployment of both the Watchman and LARIAT, necessitating the use of general endotracheal anesthesia versus monitored anesthesia care, depending on institutional preference and protocol [12]. Pre- and intra-procedural assessment of cardiac chamber and valve function can influence this anesthetic management. As with all invasive procedures, appropriate selection of anesthetic technique is paramount, as patients must be able to tolerate prolonged periods of time lying still and flat without respiratory compromise. Patients must tolerate potential discomfort from the TEE probe as well as stimulating portions of the procedure such as the groin and transseptal punctures or, in the case of the LARIAT or Sierra, sub-xyphoid access. Hemodynamic instability can occur at several points during the procedure and the anesthetic practitioner should evaluate the need for both invasive, real-time arterial blood pressure monitoring as well as adequate intravenous access to allow for rapid resuscitation of patients who are hypotensive and/or bleeding. Defibrillator pads should be applied to prepare to treat sustained arrhythmias from guidewire and catheter manipulation against the myocardium. With techniques using intracardiac devices such as the Watchman, distal embolization into the left atrium or ventricle, or into the aorta itself, is possible, as is perforation from guidewire or anchor damage to the relatively thin LAA [13]. The LARIAT device, which requires epicardial puncture, can result in inadvertent myocardial puncture, and it also carries the risk of LAA laceration or avulsion from its snare, which can result in significant pericardial effusion. Although an improved safety profile has been demonstrated with both products as more procedures are performed and operator experience and expertise improves, incomplete closure of the LAA remains a significant problem, especially in the LARIAT, where a 2016 report detailed a 7% rate of this problem [14]. As these percutaneous LAA occlusion devices have been demonstrated to be noninferior to anticoagulation in certain group of AF patients, their use will continue to be evaluated. Unfortunately, to achieve success, application of these devices may depend on patient selection and operator skills. Some complications such as severe bradycardia or arrythmias may be inevitable and should be treated actively, whereas with asystole or cardiac arrest-induced hemodynamic instability, following the Advanced Cardiovascular Life Support resuscitation protocol is warranted [12]. TEE can evaluate the extent of pericardial effusion; placing a pigtail drain can be an effective initial step. However, if pericardial effusion persists or develops to cardiac tamponade, performing surgical exploration may be necessary to assess the degree of myocardial injury and conduct definitive repair accordingly [12,15].
Expert-level use of intraprocedural TEE from either cardiology or anesthesia providers is crucial to success. The ability to supplement standard fluoroscopic views with real-time, multiplanar views of the anatomically complex LAA allows for a more complete map of ostium size, shape, depth, number of lobes, and position and correct sizing of devices. Per Mobius-Winkler, et al., the four ideal TEE views of the LAA include (a) A midesophageal (ME) four-chamber view at 0°, (b) A ME mitral commissural view at 60°, (c) A ME two-chamber view at 90°, and (d) A ME aortic valve long-axis view at 135°. A careful examination of each of these views should be undertaken to ensure guidewires and catheters are correctly positioned within the appendage at each step of the procedure [16]. X-plane and three-dimensional (3D) imaging can be helpful in selecting the appropriate location along the interatrial septum for the performance of transseptal puncture and guidance of wires for device delivery (Figure 1). After deployment, TEE can be used to evaluate leaking around seal devices, hemopericardium, pericardial effusion, tamponade, or right ventricular compression. Surgical treatments of AF continue to evolve. Unfortunately, the long-term prospective study results on LAA occlusion devices have been somewhat disappointing, with heterogeneous outcomes reported. The stroke prevention rate was only noninferior to oral anticoagulants, and procedures were associated with variable types of complications [15,17,18]. The recently developed Watchman FLX device has demonstrated encouraging short-term efficacy with a high degree of LAA sealing and low procedure compilation rates [19,20].
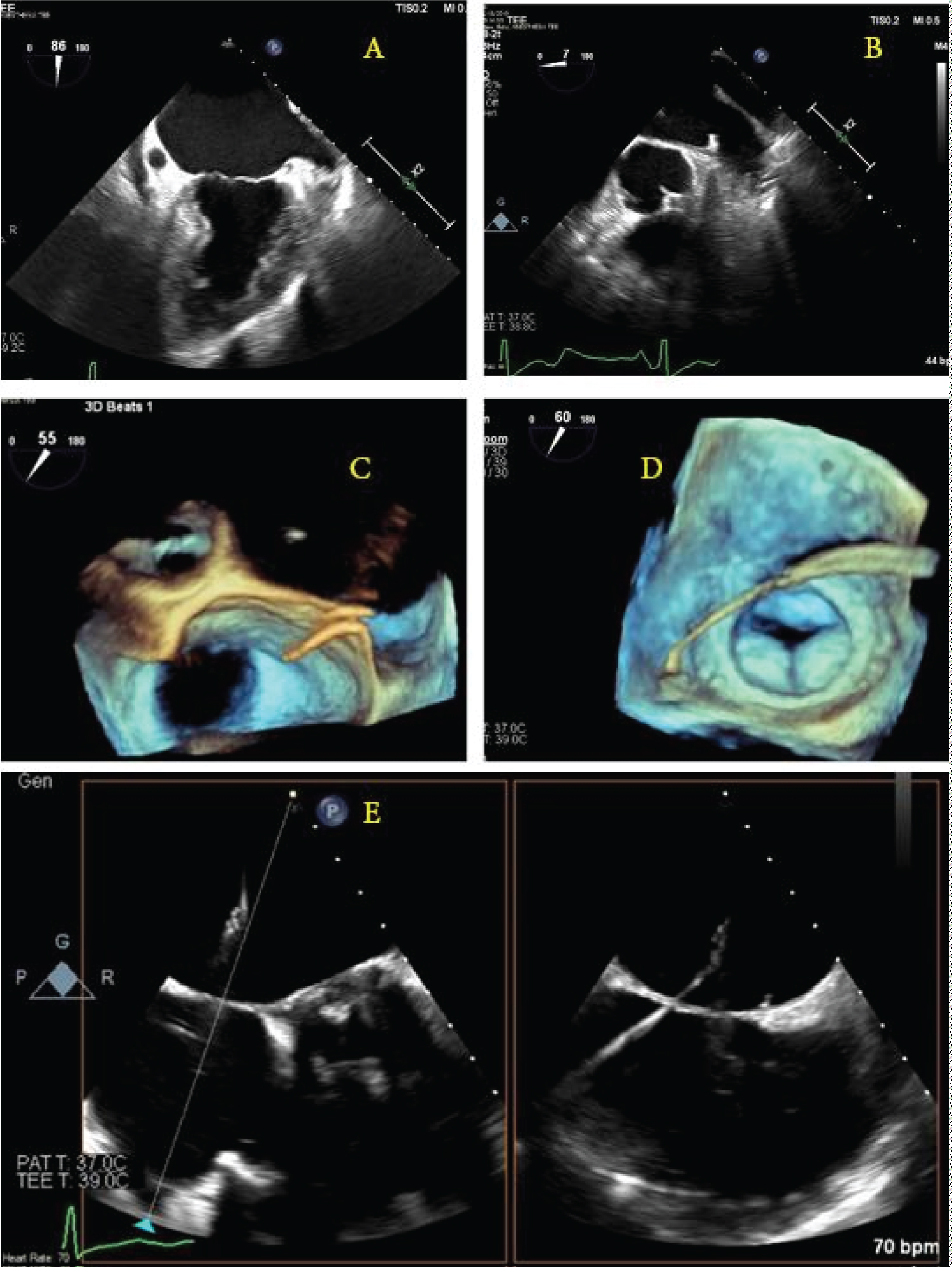
Figure 1: Schematic illustration of the guidewire punch location and direction under the direction of real-time transesophageal echocardiogram 2D/3D images. (a,b) The left atrial appendage as seen at 90° and 0°; (c) A 3D view of the wire coming across the interatrial septum; (d) A 3D image showing the wire crossing the left atrium and entering the left atrial appendage; (e) X-plane image-guidance of transseptal puncture.
Catheter Ablation Procedures for the Treatment of AF
Sleeves of myocardium extend from the left atrium into the pulmonary veins [21]; this tissue has been found to be especially arrhythmogenic [22]. Muscle bundles around the coronary sinus and great cardiac vein often contain sympathetic nerves and ganglia that can initiate or propagate abnormal electrical activity [23]. The discussion of the rotation and organization of the electrical impulses is beyond the scope of this article. In cases where the onset of AF is acute, it may be terminated with direct-current cardioversion, usually necessitating a brief general anesthetic and carrying high failure and recurrence rates. Anticoagulation is indicated even in these patients. As it is performed during open cardiac surgery, the Maze procedure, designed to create scars and disrupt conduction via freezing, radio waves, microwave, or ultrasound energy, has a morbidity and invasiveness burden greater than that of the less-invasive catheter ablation procedures [24]. These catheter-based techniques often focus on ablation or isolation of the pulmonary vein ostia, the superior vena cava, and/or the cavotricuspid isthmus via endovascular approaches. Incorporating robotic navigation or 3 D electro-anatomical mapping systems, energy is delivered via radiofrequency, cryoablation, laser, or microwave modalities. Novel therapies in development include electroporation, focused ultrasound, and external radiation, with the possible use of computed tomography or magnetic resonance imaging guidance to show the need for intracardiac access. Finally, atrioventricular node ablation with permanent pacemaker implantation removes the need for rate or rhythm control but still requires anticoagulation and carries the morbidity associated with pacemaker placement; therefore, it is reserved for special cases.
Complications Associated with Catheter Ablation Procedures
The most common complications associated with catheter ablation procedures for AF include cardiac tamponade, hemo- and/or pneumothorax, vascular access-related complications, stroke, phrenic nerve injury, pulmonary vein stenosis, atrioesophageal fistula (AEF), and death. The choice of anesthesia for these procedures should be made in collaboration with proceduralists, keeping in mind the length of the procedure and the need for minimal patient movement. One center found that general anesthesia improved success rates and shortened overall procedure duration [25], data are beginning to suggest an increased occurrence of AEF in patients who have undergone general anesthesia [26].
Atrioesophageal fistula is a life-threatening complication, further discussion is warranted. Given the proximity of the posterior left atrial wall, where the pulmonary veins reside, and the esophagus, 0.1-0.25% of AF ablations are associated with esophageal ulcerations, which can lead to AEF, pericardial-esophageal fistula, esophageal perforation, stroke, or death if diagnosis is delayed [27]. Attempts to limit energy application in the proximity of the esophagus often fail because these structures are closely related to each other anatomically. During the procedure itself, Contact Force (a large determinant of procedure success) must be balanced carefully: Too little will prevent the formation of an effective lesion, while too much can cause deep tissue injury and necrosis beyond the walls of the heart. Measurement sensors on the catheters attempt to limit excessive Contact Force and have the added benefit of expanding complete circumferential contact and improving procedural success [28]. Although most cases occur with radiofrequency ablation, esophageal injury can still occur with cryoablation, focused ultrasound, and surgical procedures. Excess energy that travels into the esophagus may contribute to ulcer formation via two mechanisms. First, this energy causes direct mucosal injury and disrupts the epithelial lining of the esophagus. Second, vagal plexus injury promotes gastroparesis and increases gastroesophageal reflux. Subsequently, these acidic gastric juices erode further into the areas of endothelial damage to cause deeper injury and ulceration [29]. In one center, post-ablation esophagogastroduodenoscopy (EGD) found an incidence of 11.6% of tissue lesions in asymptomatic patients [30].
There is considerable interest in preventing esophageal injuries during ablation procedures. Efforts to maximize the distance between the probe and the esophagus are often futile because the esophagus is highly mobile (as much as 2 cm) within the posterior mediastinum and thus can move within millimeters of the left atrium and pulmonary veins [31]. Power can be limited by the operators when performing ablations mapped to locations close to the posterior left atrial wall. One area of promise is the prophylactic post-procedure use of proton pump inhibitors to mitigate the expected esophageal injury from gastroesophageal reflux and gastroparesis [32]. Esophageal temperature probes provide feedback on when energy delivery thresholds are approaching dangerous levels (generally considered to be anything above 41° Celsius) but require general anesthesia. A patient who is unconscious cannot voice pain or discomfort, and the rigid esophageal foreign body allows for closer proximity with the ablation probe. Indeed, placing an esophageal temperature probe increases the odds ratio of esophageal injury to 16.7 [33]. For patients with temperature probes placed and a measured temperature above 41° Celsius, screening EGD can identify patients needing close follow-up.
A chest X-ray may show a widened mediastinum or pneumomediastinum; if oral contrast is used as in a fluoroscopic swallow study, a leak may be seen during the imaging series. Computed tomography scanning with intravenous and oral contrast is the best imaging study at this point and is usually only positive late in the course of the disease, with findings including pneumomediastinum or pneumopericardium (Figure 2) [34]. The morbidity and mortality of open surgical operations for repair are high, but compared to conservative medical therapy or endoscopic stenting, early surgical intervention provides the best chances of survival [35], as earlier studies have shown almost 100% mortality by endoscopic stenting of the perforation [36]. Options for surgical repair include: 1) Median sternotomy with full use of cardiopulmonary bypass, patch repair of atrial defect from within the left atrium, followed by endoscopic stenting of the esophagus; 2) Right thoracotomy with femoral-femoral bypass, patch repair of atrial defect from either within or external to the left atrium, followed by placement of a pedicled muscle flap between the esophagus and atrium [37]; and 3) Median sternotomy with full use of cardiopulmonary bypass, patch repair of the atrial defect from within the left atrium, followed by left thoracotomy for positioning of the muscle flap between the esophagus and left atrium.
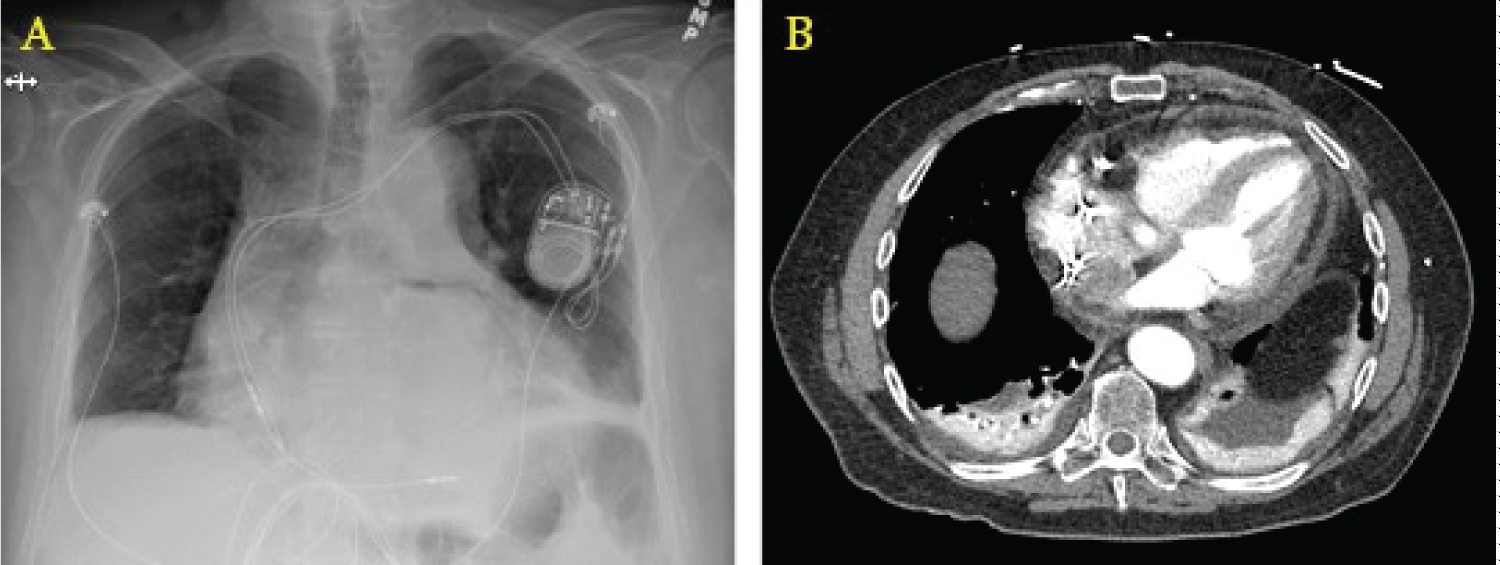
Figure 2: Radiographic findings of AEF. (a) Widened mediastinum on chest X-ray; (b) Computed tomography showing pneumopericardium with air tracking up the ascending aorta.
Conclusions
The incidence and prevalence of AF continue to increase, and this subset of the population will increasingly come under the care of an anesthesiologist. Antiarrhythmics and traditional and novel oral anticoagulants must be managed appropriately in the perioperative period. Newer procedures to definitively treat AF are in use, necessitating a specialized skillset from anesthesia personnel (non-operating room anesthesia provision, TEE skills) in unfamiliar settings (catheterization lab, hybrid suites) with many unfamiliar faces (catheterization lab personnel, pharmaceutical representatives, device company representatives). Unique and serious complications can occur during these procedures that require rapid interventions and proactive resuscitation, among them pericardial effusion/hemorrhage with tamponade, pneumothorax, mediastinitis, and AEF. Complete training and a multidisciplinary approach is necessary to safely care for these patients.
Conflicts of Interest and Source of Funding
None declared.
References
- Kloosterman M, Crijns HJGM, Van Gelder IC. Rising prevalence of atrial fibrillation in the elderly population: new challenges of geriatric cardiology. Europace. 2019;21:1451–3. doi: 10.1093/europace/euz234.
- Allan V, Honarbakhsh S, Casas JP, et al. Are cardiovascular risk factors also associated with the incidence of atrial fibrillation? A systematic review and field synopsis of 23 factors in 32 population-based cohorts of 20 million participants. ThrombHaemost. 2017;117:837–50.doi: 10.1160/TH16-11-0825
- Rogers P, Bernard ML, Madias C, et al. Current evidence-based understanding of the epidemiology, prevention, and treatment of atrial fibrillation. CurrProblCardiol. 2018;43:241–83.doi: 10.1016/j.cpcardiol.2017.06.001
- Wijffels MC, Kirchhof CJ, Dorland R, et al. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–68.doi: 10.1161/01.cir.92.7.1954
- Lip GYH, Tse HF, Lane DA. Atrial fibrillation. Lancet. 2012;379:648–61.doi: 10.1016/S0140-6736(11)61514-6
- Moussa Pacha H, Al-Khadra Y, Soud M, et al. Percutaneous devices for left atrial appendage occlusion: a contemporary review. World J Cardiol. 2019;11:57–70. doi: 10.4330/wjc.v11.i2.57
- Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–100.doi: 10.1378/chest.10-0134
- Ueberham L, Dagres N, Potpara TS, et al. Pharmacological and non-pharmacological treatments for stroke prevention in patients with atrial fibrillation. Adv Ther. 2017;34:2274–94. doi: 10.1007/s12325-017-0616-6.
- Ailawadi G, Gerdisch MW, Harvey RL, et al. Exclusion of the left atrial appendage with a novel device: early results of a multicenter trial. J Thorac Cardiovasc Surg. 2011;142:1002–9.e1.doi: 10.1016/j.jtcvs.2011.07.052
- Healey JS, Crystal E, Lamy A, et al. Left atrial appendage occlusion study (LAAOS): results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am Heart J. 2005;150:288–93.doi: 10.1016/j.ahj.2004.09.054
- Srivastava MC, See VY, Dawood MY, et al. A review of the LARIAT device: insights from the cumulative clinical experience. SpringerPlus. 2015;4:522. doi: 10.1186/s40064-015-1289-8
- Husain Z, Safavi-Naeini P, Rasekh A, et al. Anesthetic management of patients undergoing percutaneous endocardial and epicardial left atrial appendage occlusion. Semin CardiothoracVascAnesth. 2017;21:291–301.doi: 10.1177/1089253217714581
- Price MJ. Prevention and management of complications of left atrial appendage closure devices. IntervCardiol Clin. 2014;3:301–11. doi: 10.1016/j.iccl.2013.12.001.
- Lakkireddy D, Afzal MR, Lee RJ, et al. Short and long-term outcomes of percutaneous left atrial appendage suture ligation: results from a US multicenter evaluation. Heart Rhythm. 2016;13:1030–6. doi: 10.1016/j.hrthm.2016.01.022
- Jazayeri M-A, Vuddanda V, Turagam MK, et al. Safety profiles of percutaneous left atrial appendage closure devices: an analysis of the Food and Drug Administration Manufacturer and User Facility Device Experience (MAUDE) database from 2009 to 2016. J Cardiovasc Electrophysiol. 2018;29:5–13. doi: 10.1111/jce.13362.
- Möbius-Winkler S, Sandri M, Mangner N, et al. The WATCHMAN left atrial appendage closure device for atrial fibrillation. J Vis Exp. 2012;60:3671. doi: 10.3791/3671
- Caliskan E, Cox JL, Holmes DR, et al. Interventional and surgical occlusion of the left atrial appendage. Nat Rev Cardiol. 2017;14:727–43. doi: 10.1038/nrcardio.2017.107.
- Reddy VY, Doshi SK, Kar S, et al. 5-year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol. 2017;70:2964–75. doi: 10.1016/j.jacc.2017.10.021.
- Korsholm K, Samaras A, Andersen A, et al. The Watchman FLX Device: first European experience and feasibility of intracardiac echocardiography to guide implantation. JACC Clin Electrophysiol. 2020;6:1633–42. doi: 10.1016/j.jacep.2020.06.028.
- Fuernau G, Desch S, de Waha-Thiele S, et al. Arterial lactate in cardiogenic shock: prognostic value of clearance versus single values. JACC Cardiovasc Interv. 2020;13:2208–16. doi: 10.1016/j.jcin.2020.06.037.
- Nathan H, Eliakim M. The junction between the left atrium and the pulmonary veins. An anatomic study of human hearts. Circulation. 1966;34:412–42. doi: 10.1161/01.cir.34.3.412
- Chen YC, Pan NH, Cheng CC, et al. Heterogeneous expression of potassium currents and pacemaker currents potentially regulates arrhythmogenesis of pulmonary vein cardiomyocytes. J Cardiovasc Electrophysiol. 2009;20:1039–1045.doi: 10.1111/j.1540-8167.2009.01480.x
- Cabrera JA, Ho SY, Climent V, et al. The architecture of the left lateral atrial wall: a particular anatomic region with implications for ablation of atrial fibrillation. Eur Heart J. 2008;29:356–362.doi: 10.1093/eurheartj/ehm606
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–76. doi: 10.1016/j.jacc.2014.03.022
- Di Biase L, Conti S, Mohanty P, et al. General anesthesia reduces the prevalence of pulmonary vein reconnection during repeat ablation when compared with conscious sedation: results from a randomized study. Heart Rhythm. 2011;8:368–72.doi: 10.1016/j.hrthm.2010.10.043
- Martinek M, Hassanein S, Bencsik G, et al. Acute development of gastroesophageal reflux after radiofrequency catheter ablation of atrial fibrillation. Heart Rhythm. 2009;6:1457–62.doi: 10.1016/j.hrthm.2009.06.022
- Kapur S, Barbhaiya C, Deneke T, et al. Esophageal injury and atrioesophageal fistula caused by ablation for atrial fibrillation. Circulation. 2017;136:1247–55. doi: 10.1161/CIRCULATIONAHA.117.025827
- Yokoyama K, Nakagawa H, Shah DC, et al. Novel contact force sensor incorporated in irrigated radiofrequency ablation catheter predicts lesion size and incidence of steam pop and thrombus. Circ ArrhythmElectrophysiol. 2008;1:354–62.doi: 10.1161/CIRCEP.108.803650
- Shah D, Dumonceau JM, Burri H, et al. Acute pyloric spasm and gastric hypomotility: an extracardiac adverse effect of percutaneous radiofrequency ablation for atrial fibrillation. J Am Coll Cardiol. 2005;46:327–30.doi: 10.1016/j.jacc.2005.04.030
- Knopp H, Halm U, Lamberts R, et al. Incidental and ablation-induced findings during upper gastrointestinal endoscopy in patients after ablation of atrial fibrillation: a retrospective study of 425 patients. Heart Rhythm. 2014;11:574–78.doi: 10.1016/j.hrthm.2014.01.010
- Good E, Oral H, Lemola K, et al. Movement of the esophagus during left atrial catheter ablation for atrial fibrillation. J Am Coll Cardiol. 2005;46:2107–10. doi: 10.1016/j.jacc.2005.08.042
- Shaheen NJ, Stuart E, Schmitz SM, et al. Pantoprazole reduces the size of postbanding ulcers after variceal band ligation: a randomized, controlled trial. Hepatology. 2005; 41:588–94.doi: 10.1002/hep.20593.
- Müller P, Dietrich JW, Halbfass P, et al. Higher incidence of esophageal lesions after ablation of atrial fibrillation related to the use of esophageal temperature probes. Heart Rhythm. 2015;12:1464–9. doi: 10.1016/j.hrthm.2015.04.005.
- Barbhaiya C, Kumar S, Guo Y, et al. Global survey of esophageal and gastric injury in atrial fibrillation ablation: characteristics and outcomes of esophageal perforation and fistula. JACC Clin Electrophysiol. 2016;2:143–50.doi: 10.1016/j.jacep.2015.10.013.
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–444.doi: 10.1016/j.hrthm.2017.05.012.
- Mohanty S, Santangeli P, Mohanty P, et al. Outcomes of atrioesophageal fistula following catheter ablation of atrial fibrillation treated with surgical repair versus esophageal stenting. J Cardiovasc Electrophysiol. 2014;25:579–84.doi: 10.1111/jce.12386.
- Haggerty KA, George TJ, Arnaoutakis GJ, et al. Successful repair of an atrioesophageal fistula after catheter ablation for atrial fibrillation. Ann Thorac Surg. 2012;93:313–5. doi: 10.1016/j.athoracsur.2011.05.050.
Table of Contents
- Abstract
- Keywords
- Introduction
- Medical Treatment of AF and Conflict with Surgery
- Therapeutic Devices for Treatment of AF
- Catheter Ablation Procedures for the Treatment of AF
- Complications Associated with Catheter Ablation Procedures
- Conclusions
- Conflicts of Interest and Source of Funding
- Figure 1
- Figure 2
- Table 1
- References