Translational Perioperative and Pain Medicine (ISSN: 2330-4871)
ARTICLE DOI: 10.31480/2330-4871/205
Review Article | Volume 12 | Issue 1 Open Access
Cervicogenic Sleep Disorder Syndrome: Definition and Classification Recommendations
Qin Yin1#, Ming-Yue Cheng1#, Shu Wang2, Yu-E Sun3, Jin-Feng Wang4,5* and Wei Cheng6*
1The Affiliated Hospital of Xuzhou Medical University, Xuzhou 221002, P.R. China
2Yancheng Third People's Hospital, Yancheng, 224001, P.R. China
3Drum Tower Hospital Affiliated to Nanjing University Medical College, Nanjing, 210008, P.R. China
4Xuzhou Central Hospital, Xuzhou, P.R. China
5The Suzhou Hospital of the Chinese Academy of Traditional Chinese Medicine - Xiyuan Hospital
6The Affiliated Huai'an No.1 People's Hospital of Nanjing Medical University, Huai'an 223300, P.R. China
#Qin Yin and Ming-Yue Cheng
Wei Cheng, The Affiliated Huai'an No.1 People's Hospital of Nanjing Medical University, Huai'an 223300, China, Tel: +861-879-620-5791; Jin-Feng Wang, Xuzhou Central Hospital, Xuzhou, P.R. China; The Suzhou Hospital of the Chinese Academy of Traditional Chinese Medicine - Xiyuan Hospital, China, Tel: +861-816-877-9112Editor: Renyu Liu, MD; PhD; Professor, Department of Anesthesiology and Critical Care, Perelman School of Medicine at the University of Pennsylvania, Center of Penn Global Health Scholar, 336 John Morgan building, 3620 Hamilton Walk, Philadelphia, PA 19104, USA, Fax: 2153495078.
Received: June 25, 2025 | Accepted: July 23, 2025 | Published: July 26, 2025
Citation: Yin Q, Ming-Yue Cheng, Wang S, et al. Cervicogenic Sleep Disorder Syndrome: Definition and Classification Recommendations. Transl Perioper Pain Med 2025; 12(1):746-767
Abstract
Sleep disorders related to cervical diseases represent a poorly defined category of sleep disturbances closely associated with cervical musculoskeletal dysfunctions, autonomic dysregulation, and chronic pain. Despite increasing clinical recognition, an integrated pathophysiological framework remains lacking.
This review proposes a novel subtype-based framework for cervicogenic sleep disorder syndrome (CSDS), grounded in neuroanatomical, autonomic, and fascial mechanisms, to improve diagnostic clarity and therapeutic precision.
We synthesized evidence from anatomical studies, HRV-based autonomic evaluation, glymphatic flow imaging, and clinical interventions including fascial manipulation, craniosacral therapy, and acupuncture. Subtypes A–J were constructed based on shared pathomechanisms such as sympathetic overactivation, cervical dural tension, and myodural bridge dysfunction.
Each subtype demonstrates unique symptom clusters, postural profiles, and diagnostic indicators. Proposed treatments include multimodal strategies such as postural realignment, myofascial release, and autonomic modulation. Emerging technologies including wearable sleep monitoring and machine learning algorithms are also reviewed for CSDS assessment.
The CSDS subtype framework offers a clinically actionable model that bridges musculoskeletal pathology and sleep regulation. This paradigm supports targeted, integrative interventions for sleep disorders rooted in cervical dysfunction, with implications for pain medicine, rehabilitation, and digital health monitoring.
Keywords
Cervicogenic sleep disorder syndrome, Classification, Autonomic dysfunction, Fascial
Introduction
Public health burden and diagnostic blind spots of sleep disorders
The prevalence of insomnia and related sleep disturbances ranges from 10% to 30% in the general population, consistently ranking among the top three complaints in neurology, psychiatry, and primary care settings [1]. Although diagnostic criteria for insomnia have been standardized by the International Classification of Sleep Disorders, Third Edition (ICSD-3) and the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), clinical practice still relies heavily on subjective scoring systems such as the Pittsburgh Sleep Quality Index (PSQI) and the Insomnia Severity Index (ISI), as well as routine polysomnography. These tools focus primarily on 'sleep itself,' often neglecting systematic screening for somatic triggers [2].
In many cases, insomnia is not a primary disorder but a symptomatic expression of Somatic Symptom Disorder (SSD). When accompanied by chronic pain, gastrointestinal discomfort, or cardiovascular sympathetic overactivation, insomnia may reflect broader systemic dysfunction. Misclassifying such cases as primary insomnia may result in missed opportunities for early intervention and lead to suboptimal treatment outcomes [2].
Bidirectional amplification between somatic symptom disorder and sleep disturbance
Recent longitudinal cohort studies and systematic reviews have demonstrated a bidirectional causal relationship between chronic spinal pain, musculoskeletal dysfunction, and insomnia. Sleep disturbances may lower pain thresholds and enhance pain memory, while persistent pain can disrupt sleep continuity via sympathetic–thalamic-cortical circuits [3,4]. Heightened limbic sensitivity to pain-arousal inputs is central to the delayed sleep onset and frequent awakening phenotype. From the perspective of neuroregulation, sustained somatic sensory input can maintain the sleep-regulatory network in a hypervigilant state [1,2].
Independent role of cervical dysfunction in sleep disturbance
A growing body of evidence indicates that, for a subset of patients, insomnia is not primarily driven by emotional, environmental, or neurological disorders, but rather is closely associated with structural or functional cervical abnormalities [4,5]. These cases may represent an independent subtype of insomnia with a cervicogenic origin, whose pathophysiological features differ significantly from traditional primary insomnia or psychiatric comorbid insomnia [5].
Association between cervical degeneration and sleep disturbances : The majority of patients with cervical spondylosis present with sleep disturbances [5-7]. Surgical correction of cervical abnormalities often leads to notable improvements in sleep symptoms, with nearly half of patients reporting complete remission. Baseline risk factors for unsuccessful sleep recovery postoperatively include smoking, osteoarthritis, radicular symptoms, and chronic neck pain [6,7]. Biomechanical factors also contribute to the association between cervical degeneration and disordered sleep [6]. Data from the Taiwan National Health Insurance Research Database further indicate a strong correlation between cervical spondylosis and obstructive sleep apnea (OSA) [8].
Over 60% of hospitalized patients with cervical spondylosis report sleep issues upon admission, typically presenting with a triad of difficulty initiating sleep, frequent awakenings, and non-restorative sleep. Significant improvements in sleep parameters following structural cervical correction support the hypothesis that cervical anomalies may constitute an independent and modifiable etiological factor [6,7,9].
Neck pain as an independent predictor of poor sleep quality: Neck and shoulder pain in middle-aged women has been shown to be significantly associated with poor sleep quality, even after adjusting for confounding factors such as anxiety, depression, and lifestyle habits [10]. This suggests that cervical myofascial tension may independently disrupt sleep architecture.
Based on data from the longitudinal Norwegian HUNT study, Uhlig et al. reported that individuals with insomnia had a 1.34-fold increased risk of developing chronic musculoskeletal neck pain (95% confidence interval [CI]: 1.10-1.63). Insomnia was also linked to a higher risk of pain in multiple anatomical regions, indicating its potential role as a predictor of chronic pain onset [3].
Chronic neck pain and sleep disturbances may share genetic and environmental predispositions. In monozygotic twins, if one reported neck pain, the likelihood of the co-twin experiencing sleep disorders was significantly higher than expected by chance [11]. This supports the hypothesis that cervicogenic sleep disturbance may have reproducible and cross-individual biomarker traits.
Cervical dystonia and its association with sleep quality: In a prospective study of 54 patients with Cervical Dystonia (CD) and 55 healthy controls, Eichenseer, et al. found that individuals with CD reported significantly poorer sleep quality, even after controlling for depression, anxiety, and benzodiazepine use (F = 5.62, P = 0.020) [12].
These patients exhibited specific alterations in sleep architecture and responded positively to targeted interventions [13-15], highlighting the potential for sleep-directed therapies in CD populations.
Neural axons, hemodynamics, and sleep regulation : Cervical compression may chronically activate sympathetic tone via a neuroanatomical cascade involving the vagus nerve–locus coeruleus-hypothalamus pathway [16]. Analysis of a clinical outcomes database including 4,245 patients demonstrated that individuals with cervical spondylosis exhibited significantly elevated preoperative Respiratory Disturbance Index (RDI) and Epworth Sleepiness Scale (ESS) scores compared to matched controls (P < 0.001), with a 42% symptomatic remission rate within 3 months post-decompression [5].
Positron emission tomography-cerebral blood flow (PET-CBF) studies in patients with chronic whiplash injury and internal jugular vein (IJV) stenosis suggest that spinal gliosis and central hypoperfusion may collectively induce energy crises within sleep–wake regulatory centers [17,18], reinforcing the hypothesized pathophysiological cascade: cervical degeneration → impaired central arousal regulation → fragmented sleep.
Further evidence from patients with IJV and cerebral venous sinus stenosis reveals that venous stenting leads to significant improvements in symptoms such as headache, tinnitus, visual impairment, papilledema, and insomnia. During an average follow-up of 30.5 months, 95.5% of patients maintained adequate venous patency without serious complications [19].
The 'cervical-brain-autonomic-sleep' cross-system mechanism chain : The pathological process by which cervical dysfunction affects sleep can be conceptualized as a six-tiered cascading mechanism involving cervical structures, brainstem networks, autonomic regulation, and sleep circuits. These levels can operate independently or interactively, ultimately producing clinical phenotypes such as sleep fragmentation and circadian rhythm disruption.
(1) The peripheral structural–biomechanical level is the initiating tier of this cascade. Cervical spondylosis, deep muscle and fascial tension anomalies, myodural bridge traction, and instability at the C0-C2 segments may produce persistent nociceptive and proprioceptive input at the dorsal horn of the spinal cord [3,20].
(2) Neural-Fascial-Hemodynamic-Glymphatic Coupling
Persistent traction of the myodural bridge, impaired venous and lymphatic drainage, and abnormal vertebral arterial perfusion may disrupt cerebrospinal fluid (CSF) flow and contribute to regional hypoperfusion [21,22]. Impaired cranial venous outflow can elevate intracranial pressure and obstruct CSF circulation, subsequently reducing the clearance efficiency of the glymphatic system and facilitating the accumulation of neurotoxic waste [23].
In patients with idiopathic intracranial hypertension (IIH), the severity of transverse sinus stenosis is highly correlated with glymphatic dysfunction. This suggests that venous reflux disturbances may impair CSF–interstitial fluid (ISF) exchange and lead to intracerebral toxin accumulation [24].
(3) Medullary Integration and Reticular Dysregulation
In this tier, disinhibition of the medullary reticular formation, including disrupted coupling between the pre-Bötzinger complex and locus coeruleus, has been observed, along with reactive gliosis. These ascending neural discharges lower arousal thresholds and destabilize respiratory rhythms [25,26].
(4) Thalamocortical Disintegration
Excessive thalamic firing impairs theta–gamma coupling, amplifies nociceptive–arousal circuits, and suppresses deep slow-wave activity (N3), resulting in sleep fragmentation [27,28].
(5) Neuroendocrine-Immune Interface
With continued ascending activation, the hypothalamic–pituitary–adrenal (HPA) axis becomes hyperactivated. Autonomic balance, as reflected in heart rate variability (HRV), shifts toward low vagal and high sympathetic dominance. Inflammatory mediators are also upregulated, leading to reductions in both the quality and quantity of deep sleep [29,30].
(6) Circadian Disruption and Systemic Crosstalk
At the systemic level, parasympathetic tone is further suppressed, melatonin secretion is delayed, and circadian rhythm sleep-wake disorders (CRSWD) may emerge. Clinical signs include elevated low-frequency/high-frequency (LF/HF) ratios and prolonged sleep latency [31,32]. Inflammation and metabolic dysregulation contribute to a vicious cycle of disturbed sleep, pain, and mood alterations [33-35].
Fanielle emphasized the link between somatic symptom disorder (SSD) and sleep disturbance [33], underlining the need for a biopsychosocial approach. Chronic cervical pain, inflammation, and sleep disruption may dysregulate the hypothalamic-pituitary-target gland axes (hypothalamic-pituitary-adrenal (HPA), hypothalamic-pituitary-thyroid (HPT), and hypothalamic-pituitary-ovarian (HPO) ) and the autonomic nervous system (ANS), accompanied by systemic inflammation, thereby disturbing hormonal, sleep, and immune homeostasis across multiple systems [36-40].
This six-tiered pathophysiological model-spanning cervical dysfunction, brainstem-autonomic imbalance, neuroendocrine–immune amplification, and circadian breakdown-offers a comprehensive framework for stratified diagnosis and targeted interventions. Cervical pathology is not merely a local disorder but can profoundly affect sleep through multisystem interactions. Thus, conventional sleep phase or architecture-based classifications may be insufficient, necessitating integrative diagnostic and treatment strategies.
Limitations of current diagnostic systems and the need for a new paradigm
Sateia, et al. noted that the third edition of the International Classification of Sleep Disorders (ICSD-3) significantly revised the classification of insomnia by merging 'primary' and 'secondary' insomnia into a unified diagnosis of chronic insomnia disorder, emphasizing its status as an independent disease entity [41]. However, this approach lacks specificity for medically-induced insomnia, such as cases secondary to cervical spine pathology, posing challenges for identification and management in practice.
In clinical settings, the evaluation of cervical-related sleep disorders varies by specialty, often leading to fragmented data and uncoordinated care. For example, orthopedic surgeons emphasize radiographic anomalies, neurologists focus on polysomnographic indices, and rehabilitation specialists rely on joint range of motion and pain scales. This siloed approach limits interdisciplinary collaboration and results in disjointed patient care [42].
Furthermore, imaging findings of spinal degeneration are prevalent in asymptomatic individuals, making it difficult to determine clinical significance based on radiological evidence alone [43]. This underscores the importance of correlating imaging with clinical symptoms in diagnosis.
Limitations of current artificial intelligence in diagnosis and the need for a new framework
Despite increasing applications of artificial intelligence (AI) in medicine, its development is constrained by inconsistencies in labeling. For example, disorders like cervical spondylosis and insomnia are typically coded independently, without contextual linkage, thereby limiting AI’s ability to detect or predict cervicogenic sleep disorders [44].
Moreover, current diagnostic frameworks lack integration of complex pathophysiological mechanisms, restricting AI performance in multifactorial conditions [45].
Thus, there is an urgent need to construct a mechanism-based diagnostic paradigm that harmonizes interdisciplinary standards and supports precision subtyping and personalized treatment strategies. This would improve patient outcomes and establish a robust data foundation for future AI and big-data integration in sleep medicine.
Necessity and potential value of defining cervical-related sleep disorders
Academic originality : The proposed concept of 'cervical-related sleep disorders' addresses a longstanding gap in the International Classification of Sleep Disorders, Third Edition (ICSD-3) regarding organic dysfunctions at the neck-insomnia interface [46]. This innovative definition not only extends the research frontier between cervical spine disorders and sleep medicine but also provides a theoretical foundation for disease stratification and targeted intervention.
Clinical applicability: By integrating structural assessments (e.g., MRI, musculoskeletal ultrasound, functional MRI) with physiological evaluations (e.g., polysomnography, heart rate variability [HRV] analysis, circadian rhythm monitoring) [47,48], a dual-level, multilayer diagnostic framework enables precise screening, classification, and tiered management of these patients-addressing fragmentation in current clinical pathways.
Research scalability: This concept naturally integrates multiple disciplines, including neuroscience, musculoskeletal rehabilitation, hemodynamics, and immunometabolism [49]. Future research may utilize multimodal data-including imaging, physiological signals, and blood biomarkers-combined with machine learning to develop individualized models for risk prediction and therapeutic intervention [50].
Therapeutic innovation : Regarding the mechanisms linking cervical spine disorders and autonomic nervous system imbalance, the integration of minimally invasive decompression techniques (e.g., neuroendoscopic procedures) [51], fascial release therapies [52], sympathetic nerve blocks [53], neuromodulation devices such as spinal cord stimulators [54], and digital therapeutics [55] offers a promising avenue to transcend the conventional binary framework for insomnia treatment, which predominantly relies on pharmacological agents and cognitive behavioral therapy for insomnia (CBT-I) [56].
Public health significance : For high-risk occupational groups-such as office workers engaged in prolonged desk work-integrated screening for cervical abnormalities and sleep dysfunction could enable early identification and intervention [57]. This has significant public health implications, potentially disrupting the chronic pain-insomnia-cardiovascular disease cycle and reducing long-term healthcare burden.
Definition of Cervicogenic Sleep Disorder Syndrome
Definition
Growing evidence from epidemiological, imaging, and interventional studies indicates that cervical-related factors have evolved from incidental observations into traceable, quantifiable, and modifiable contributors to sleep disorders. The proposed concept of cervical-related sleep disturbance aligns with the broader trend toward precision medicine and cross-system integration, offering a shared framework for mechanistic research, diagnostic standardization, AI-driven decision support, and multidisciplinary care.
We define Cervicogenic Sleep Disorder Syndrome (CSDS) as a multisystem dysregulation syndrome initiated by cervical pathology, subsequently affecting the autonomic nervous system (ANS), the thalamo-brainstem sleep regulatory network, and the neuroendocrine-immune axis.
CSDS may involve the following structural and functional abnormalities:
(1) Abnormal tension in the upper cervical myofascial complex-such as the suboccipital muscles and sternocleidomastoid-may impair the myodural bridge (MDB), disrupting cerebrospinal fluid (CSF) dynamics and brainstem regulation [58].
(2) Cervical degeneration or deep fascial dysfunction may induce biomechanical imbalance, chronic pain, and sympathetic ganglion compression [59].
(3) Persistent cervicogenic pain may amplify nighttime awakenings and suppress N3 sleep via thalamo-cortical arousal chains [60].
(4) Neurocompression and brainstem dysfunction (e.g., C0-C2 instability causing medullary compression) may reduce arousal thresholds and disrupt respiratory rhythms [61].
(5) Vertebral arterial insufficiency, impaired venous/lymphatic outflow [62,63], and glymphatic dysfunction [24] may impair metabolic waste clearance, reduce delta wave activity, and cause HRV imbalance.
(6) MDB disconnection may abruptly reduce CSF flow and compress the brainstem's anteroposterior axis, even at rest [64].
(7) Abnormal fascial tension in the cervical region and disruption of myofascial anatomical continuity with the temporomandibular muscles may contribute to temporomandibular disorders (TMD) and nocturnal bruxism, resulting in sleep fragmentation [65]. Cervical muscle tone dysfunction-such as dystonic torticollis or hypertonicity of suboccipital muscles-can impair proprioceptive feedback and trigger hyperactivation of the central arousal system [66].
(8) ANS dysregulation, especially sympathetic–parasympathetic imbalance, is reflected by elevated LF/HF ratios or reduced HRV [67].
(9) Thalamo-medullary desynchronization impairs corticothalamic-brainstem rhythmic coherence and weakens arousal threshold regulation [68].
(10) Hyperactivation of the hypothalamic-pituitary-adrenal (HPA) axis and GABAergic imbalance may lead to sleep fragmentation, nocturnal awakenings, and blunted circadian inflammation patterns [69].
(11) Circadian misalignment and systemic reactivity may present as delayed melatonin release, sleep-wake cycle disruption, comorbid affective symptoms, and cognitive decline [70].
CSDS, as a novel interdisciplinary pathophysiological construct, elucidates how cervical spine and associated soft tissue abnormalities may systematically disrupt sleep architecture and function. It adds an anatomically grounded subtype to the classification of insomnia and supports future work in mechanism-based research, AI-assisted diagnosis, and multimodal therapeutic strategies.
Clinical Subtypes of CSDS
CSDS can be divided into the following ten mechanistic subtypes:
CSDS-A
Sympathetic Overactivation Subtype (Figure 1)
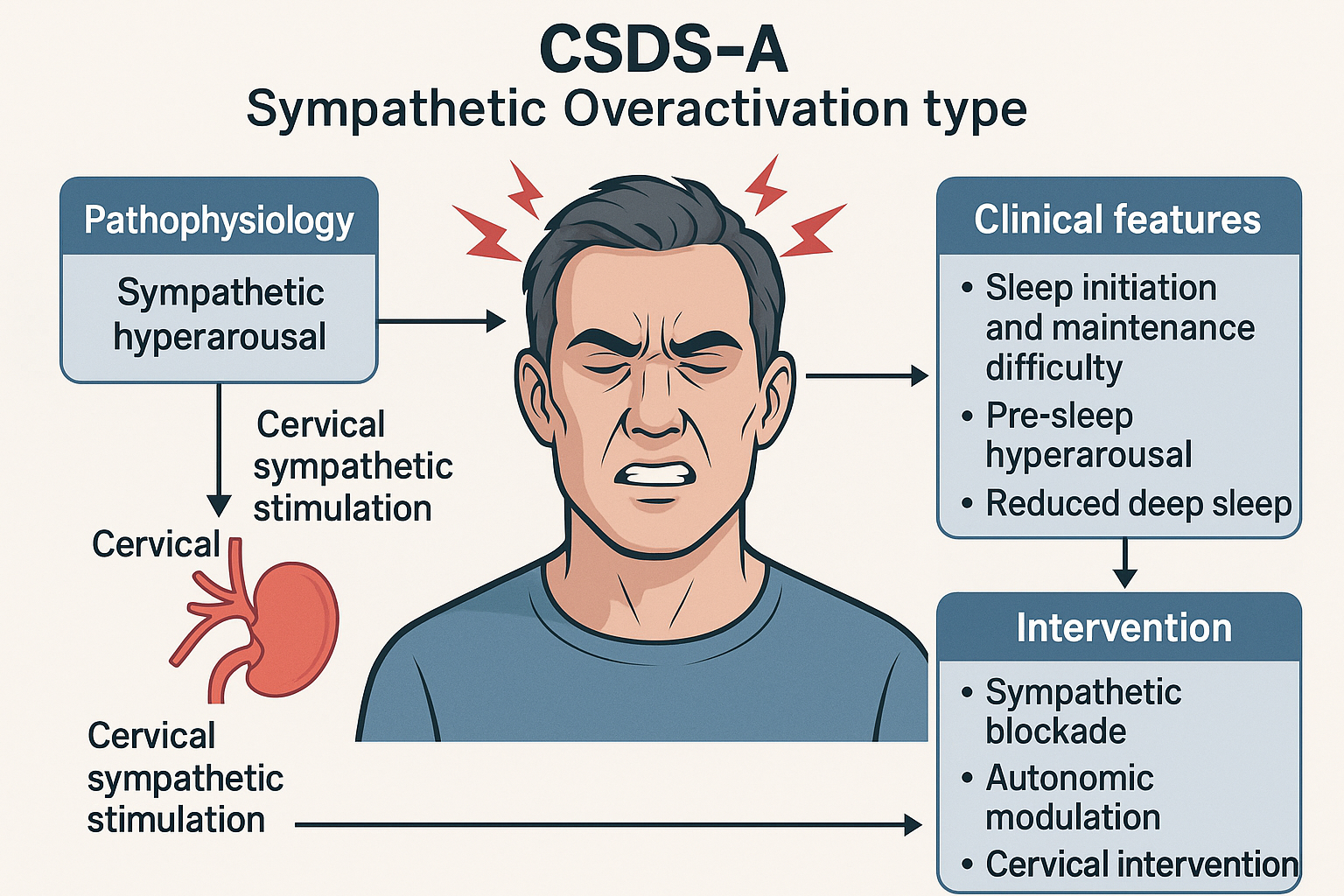
Figure 1:
Schematic diagram of CSDS-A type (Sympathetic Overactivation Type).
This diagram illustrates the pathophysiological mechanisms, clinical features, and interventional strategies of CSDS-A. Excessive cervical sympathetic stimulation leads to sympathetic hyperarousal, disrupting sleep initiation and maintenance. Key interventions target autonomic dysregulation.
Abbreviations and Explanations:
CSDS-A: Cervicogenic Sleep Disorder Syndrome - Type A; Sympathetic Overactivation: A subtype characterized by cervical-driven sympathetic hyperarousal; Cervical Sympathetic Stimulation: Functional or structural excitation of the cervical sympathetic trunk or ganglia; Sympathetic Blockade: Pharmacologic or interventional suppression of sympathetic output (e.g., stellate ganglion block); Autonomic Modulation: Interventions aimed at rebalancing the autonomic nervous system (e.g., HRV biofeedback, vagus nerve stimulation); Cervical Intervention: Manual or procedural therapies targeting the cervical spine or musculature
The core mechanism of CSDS-A involves excessive activation of the sympathetic nervous system (SNS), leading to difficulty falling asleep, heightened pre-sleep arousal, and poor sleep maintenance. Patients with objectively short sleep duration often exhibit reduced high-frequency heart rate variability (HF-HRV) and elevated low-frequency/high-frequency (LF/HF) ratios on polysomnography, indicating diminished parasympathetic and enhanced sympathetic activity [71].
Such patients demonstrate lower arousal thresholds, reduced slow-wave sleep (SWS), and increased micro-arousals-hallmarks of sustained sympathetic hyperarousal and autonomic imbalance. This aligns with the 'hyperarousal model' of insomnia [72]. Stimulation of cervical sympathetic ganglia, such as through degenerative cervical spondylosis, may lead to persistent SNS excitation, interfering with both sleep initiation and continuity [73]. Targeted management for CSDS-A should address sympathetic regulation through structural interventions and autonomic assessment to enable personalized treatment.
CSDS-B
Vagal Suppression and Circadian Dysregulation Subtype (Figure 2)
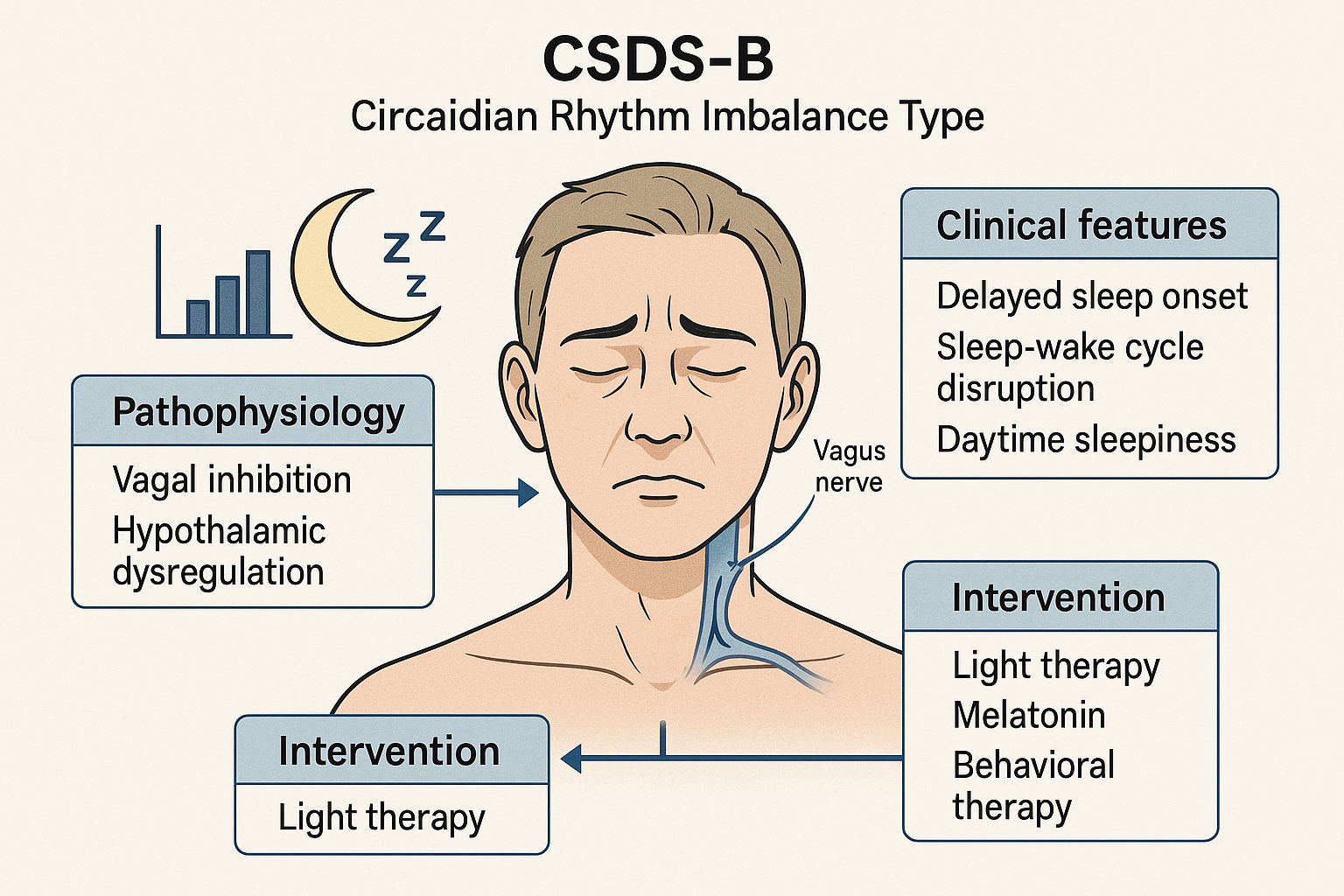
Figure 2:
Schematic diagram of CSDS-B type (Circadian Rhythm Imbalance Type).
This diagram illustrates the pathophysiology, clinical features, and treatment strategies of CSDS-B. Vagal inhibition and hypothalamic dysregulation disrupt the circadian rhythm, leading to delayed sleep onset and daytime somnolence. Interventions focus on circadian entrainment via light therapy and neurohormonal modulation.
Abbreviations and Explanations:
CSDS-B: Cervicogenic Sleep Disorder Syndrome - Type B; Circadian Rhythm Imbalance Type: A subtype characterized by disturbed biological clock alignment; Vagal Inhibition: Suppression or hypoactivity of the vagus nerve, impairing autonomic balance and circadian signalling; Hypothalamic Dysregulation: Impairment in the suprachiasmatic nucleus and hypothalamic centers that regulate sleep-wake cycles; Light Therapy: Exposure to artificial light (typically 2,500-10,000 lux) to realign circadian rhythm; Melatonin: Endogenous hormone used therapeutically to phase-shift circadian cycles; Behavioral Therapy: Includes cognitive-behavioral therapy for insomnia (CBT-I) and sleep hygiene practices.
This subtype is characterized by parasympathetic insufficiency and circadian rhythm misalignment, commonly presenting as delayed or irregular sleep–wake cycles. Clinical features include prolonged sleep onset latency, delayed melatonin secretion, and coexistence of nighttime insomnia with daytime hypersomnolence-resembling delayed sleep phase syndrome (DSPS) [74].
The core issue lies in insufficient vagal tone, impairing endogenous circadian synchronization. These patients are often misdiagnosed as having simple sleep initiation difficulties, despite the underlying phase shift pathology [74,75].
In CSDS-B patients, cervical lesions may disrupt hypothalamic–pituitary axis function, altering melatonin release and further destabilizing circadian regulation [76]. Management strategies should incorporate light therapy, exogenous melatonin supplementation, and behavioral approaches to restore biological timing and improve both nocturnal sleep and daytime alertness.
CSDS-C
Pain-Induced Arousal Subtype (Figure 3)
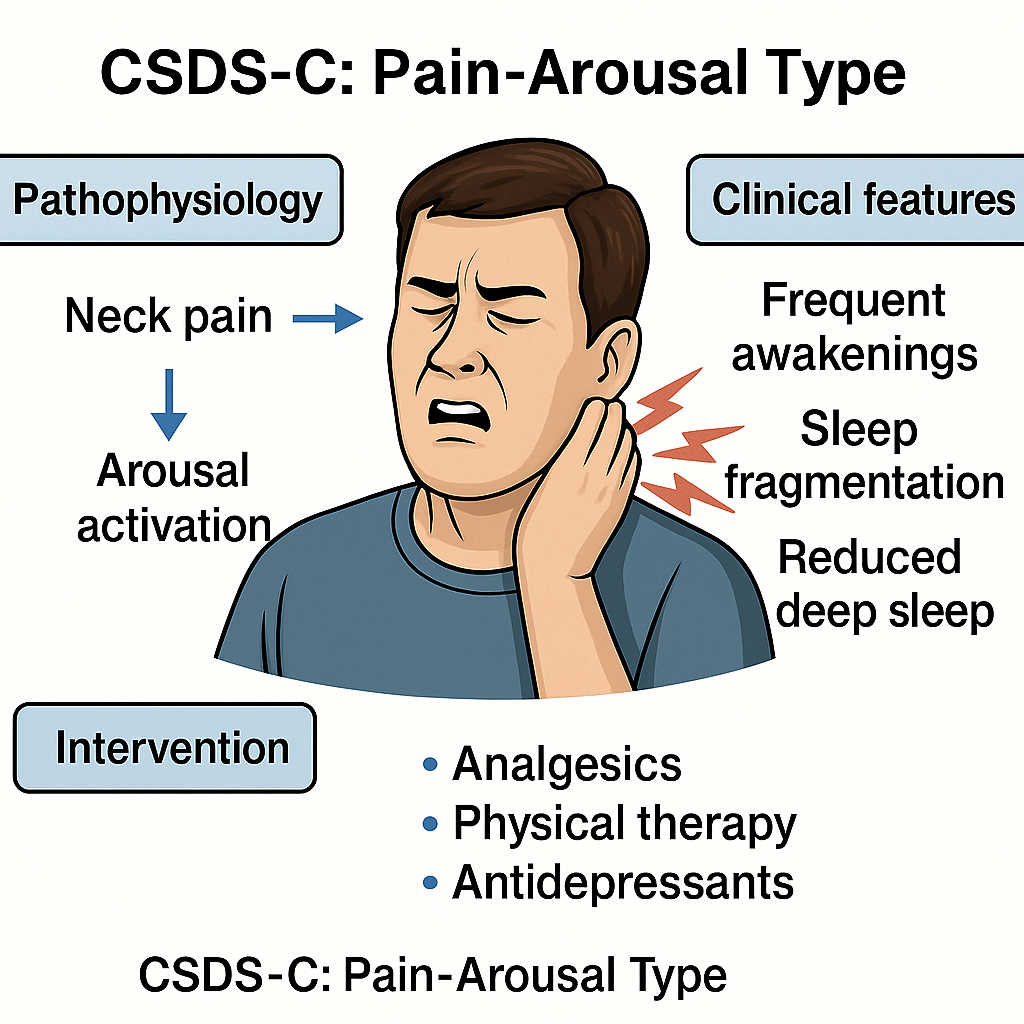
Figure 3:
Schematic diagram of CSDS-C type (Pain-Arousal Type).
This diagram illustrates the clinical and pathophysiological features of CSDS-C. Chronic or recurrent neck pain activates ascending arousal systems, leading to sleep fragmentation, frequent awakenings, and reduced deep sleep. Interventions focus on pain relief, neuromodulation, and behavioral stabilization.
Abbreviations and Explanations:
CSDS-C: Cervicogenic Sleep Disorder Syndrome - Type C; Pain-Arousal Type: A subtype characterized by nociceptive input from the cervical spine triggering central arousal; Arousal Activation: Stimulation of ascending reticular activating systems (ARAS), often measured via EEG microarousals or wake index; Analgesics: Pharmacological agents used for pain control (e.g., NSAIDs, muscle relaxants, acetaminophen); Physical Therapy: Manual therapy, postural correction, cervical mobilization targeting cervical musculoskeletal dysfunction; Antidepressants: Agents with dual analgesic and sedative properties (e.g., amitriptyline, duloxetine) used for comorbid insomnia and pain modulation
CSDS-C is marked by sleep maintenance insomnia triggered by cervical pain. Patients typically fall asleep without difficulty but experience repeated nocturnal awakenings due to pain, resulting in sleep fragmentation and reduced deep sleep. Common triggers include chronic cervical disorders such as degenerative changes and cervicogenic headaches [77].
Persistent pain signals elevate cortical arousal and interfere with sleep continuity. Twin studies have shown a strong association between chronic neck pain and poor sleep quality, independent of genetic factors-suggesting an environmental and symptomatic basis for the link [78].
Furthermore, chronic pain may exacerbate insomnia indirectly through depressive pathways, highlighting the dual direct and affective contributions of pain to sleep disturbance [79,80].
CSDS-D
Cervical Dystonia-Related Sleep Dysregulation (Figure 4).
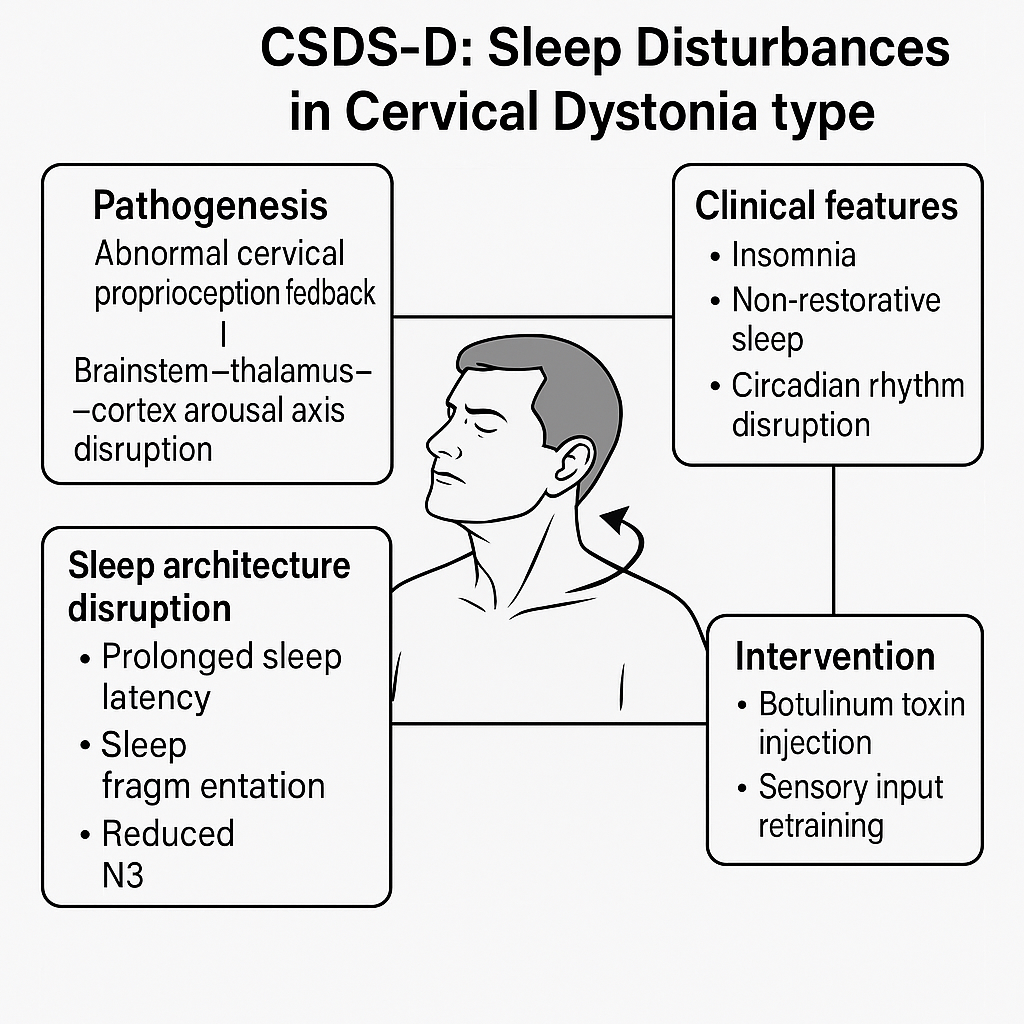
Figure 4:
Schematic diagram of CSDS-D type (Sleep Disturbances in Cervical Dystonia Type).
This figure illustrates the neurophysiological mechanisms and clinical characteristics of CSDS-D. Abnormal proprioceptive feedback from cervical dystonia disrupts the ascending arousal system from brainstem to cortex, resulting in non-restorative sleep and circadian rhythm instability. Treatment involves neurotoxin injection and proprioceptive retraining.
Abbreviations and Explanations:
CSDS-D: Cervicogenic Sleep Disorder Syndrome - Type D; N3: Non-Rapid Eye Movement Sleep Stage 3 (also referred to as slow-wave sleep), representing the deepest stage of sleep critical for restoration; Botulinum Toxin: Neurotoxin commonly used for neuromuscular blockade in cervical dystonia; Proprioception Feedback: Sensory input from muscle spindles and joint receptors informing central nervous system about position and movement;
Brainstem-thalamus-cortex Arousal Axis: A hierarchical neural system governing vigilance, sleep-wake transition, and sensory integration; Sleep Fragmentation: The interruption of sleep continuity by microarousals or awakenings, often reducing sleep efficiency; Circadian Rhythm: The endogenous 24-hour biological clock regulating sleep-wake cycles, often disrupted in dystonic conditions
CSDS-D, or sleep disorder related to cervical dystonia (SDCeD), is a central sleep regulation syndrome driven by persistent, asymmetric, or hyperactive cervical muscle tone. Key symptoms include insomnia, non-restorative sleep, and circadian rhythm disruption [81,82].
Mechanistically, abnormal proprioceptive input from the neck disturbs the brainstem-thalamic-cortical arousal network, leading to locus coeruleus-sympathetic activation, increased arousal, and suppression of SWS [83-85]. These physiological disruptions manifest as prolonged sleep latency, fragmented sleep, and reduced N3 sleep.
Preliminary clinical evidence supports this model: CSDS-D patients exhibit distinct sleep architecture features (e.g., SWS suppression, increased awakenings), demonstrable physiological abnormalities, and responsiveness to targeted interventions such as botulinum toxin injection and sensory retraining [83-85].
CSDS-E type
Myodural Tensile Dysregulation Subtype (Figure 5)
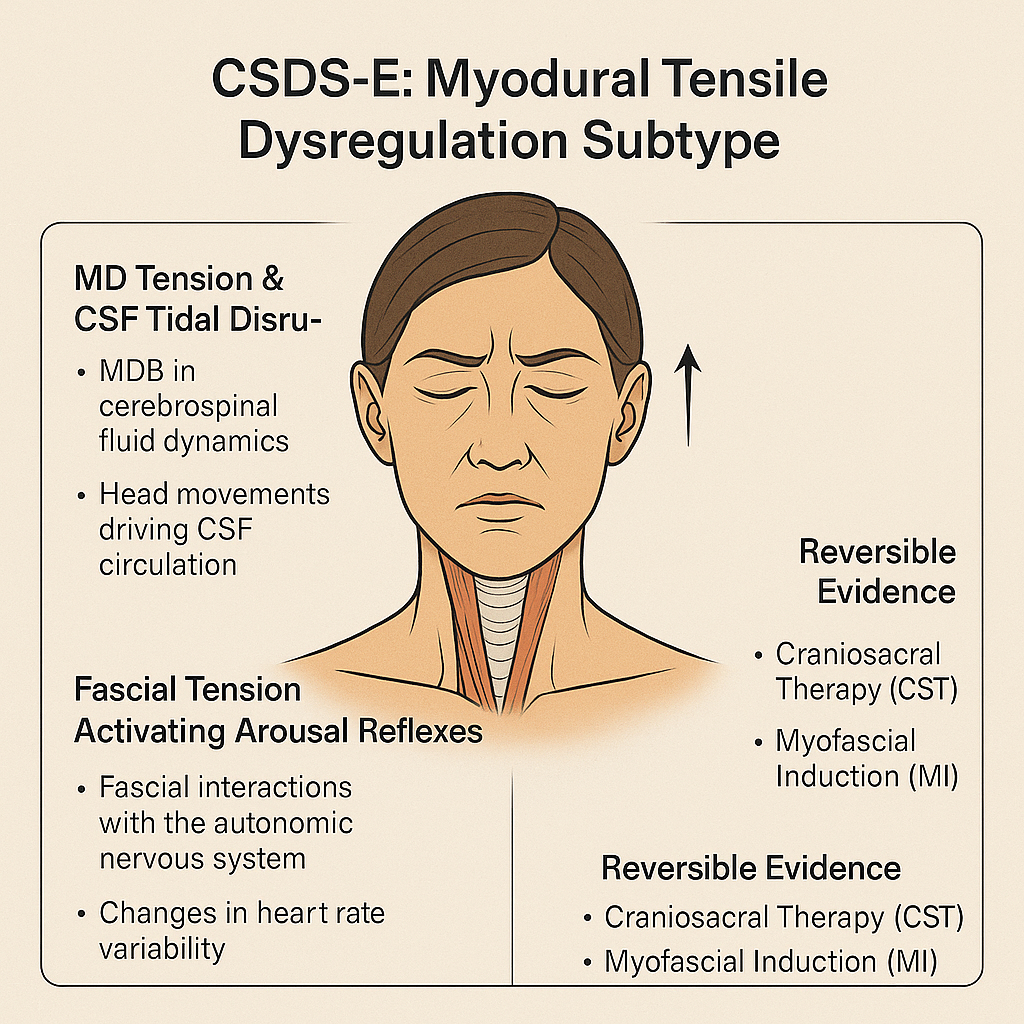
Figure 5:
Schematic diagram of CSDS-E type (Myodural Tensile Dysregulation Subtype).
This diagram illustrates the hypothesized role of myodural bridge (MDB) tension and cerebrospinal fluid (CSF) circulation disruption in sleep disturbance. The fascial system’s biomechanical influence on the autonomic nervous system, particularly via arousal reflexes and heart rate variability (HRV), is highlighted. Reversible therapeutic evidence includes craniosacral therapy (CST) and myofascial induction (MI).
Abbreviations and Explanations:
CSDS-E: Cervicogenic Sleep Disorder Syndrome - Type E; MDB: Myodural Bridge: Connective tissue linking suboccipital muscles to spinal dura mater; CSF: Cerebrospinal Fluid: A clear fluid surrounding the brain and spinal cord involved in mechanical cushioning and metabolic regulation; CST: Craniosacral Therapy - a gentle manual therapy aiming to relieve tension in the membranes and fluids surrounding the central nervous system; MI: Myofascial Induction - a manual therapy focusing on the release of fascial restrictions; HRV: Heart Rate Variability - a measure of autonomic nervous system function reflecting balance between sympathetic and parasympathetic tone; Autonomic Nervous System (ANS): The part of the nervous system regulating involuntary physiological processes including heart rate, blood pressure, and digestion
The CSDS-E subtype (Myodural Tensile Dysregulation Subtype) describes a form of sleep disorder associated with persistent tensile stress exerted on the myodural bridge (MDB) by the craniocervical myofascial chains (e.g., suboccipital muscles, sternocleidomastoid, atlantoaxial muscles) at the C0-C2 junction [86-88]. This mechanical load may disrupt cerebrospinal fluid (CSF) dynamics and arousal-related autonomic pathways, resulting in sleep architecture disruption and increased awakening frequency.
Key mechanisms of CSDS-E include:
MDB tension and CSF flow disruption : The MDB is a key anatomical linkage between cranial muscles and the dura mater [86], implicated in modulating CSF circulation. Hypertrophy of suboccipital muscles may raise intracranial pressure (ICP), while surgical disconnection of the MDB can lower ICP [87], suggesting a regulatory role of MDB in CSF hydrodynamics. Head nodding movement significantly affects CSF flow at the craniovertebral junction, with MDB traction acting on the dural sac during such movement [88], emphasizing its role in CSF propulsion.
Glymphatic impairment and morning brain fog : Slow-wave sleep (SWS, N3 stage) is critical for efficient clearance of brain metabolites [89]. During this phase, CSF enters the parenchyma via perivascular spaces and exchanges with interstitial fluid (ISF) to remove waste products like β-amyloid [90]. Reduced slow-wave activity impairs glymphatic clearance, potentially contributing to subjective symptoms such as "morning brain fog" [91,92]. Studies confirm that MDB influences CSF production and reabsorption rates [88,93], indicating that C0-C2 myofascial tension could impair glymphatic flow and metabolite elimination.
Fascial tension and autonomic-arousal reflex : The fascial system interacts with the autonomic nervous system (ANS). Mechanical tension in fascia may modulate neural function [94]. Altered heart rate variability (HRV), such as elevated LF/HF ratio, in patients with myofascial pain suggests autonomic dysfunction involvement [95].
Therapeutic evidence : Craniosacral therapy (CST) has demonstrated potential for alleviating chronic pain, improving function and quality of life [96]. A 12-week intervention in fibromyalgia syndrome (FMS) patients, receiving weekly 45-minute CST sessions, led to significant improvements in Pittsburgh Sleep Quality Index (PSQI) scores, with a mean reduction of 5.44 points (p = 0.001) [97].
The presence of myofascial trigger points (MTrPs) correlates with pain intensity, dysfunction, and poor sleep quality in mechanical neck pain patients. Higher MTrP counts and worse PSQI scores have been observed in patients compared to healthy controls [98].
These findings support the utility of CST and myofascial interventions (MI) in sleep enhancement among individuals with cervical disorders. CST improves rhythmic regulation of the craniosacral system [97], whereas MI alleviates fascial restrictions and muscular dysfunction [98].
For patients exhibiting the triad of "morning head pressure, SWS loss, and abnormal HRV," dynamic CSF flow evaluation at the C0-C2 region is recommended. Complementary assessments via polysomnography (PSG) and HRV analysis help evaluate sleep structure and ANS integrity. Additionally, the impact of respiratory patterns on CSF flow should be considered [99]. Personalized treatment should integrate breath retraining, craniosacral modulation, fascial release, and myofascial bridging techniques to optimize sleep and autonomic outcomes.
CSDS-F subtype
Temporomandibular Disorder-Driven CSDS (Figure 6)
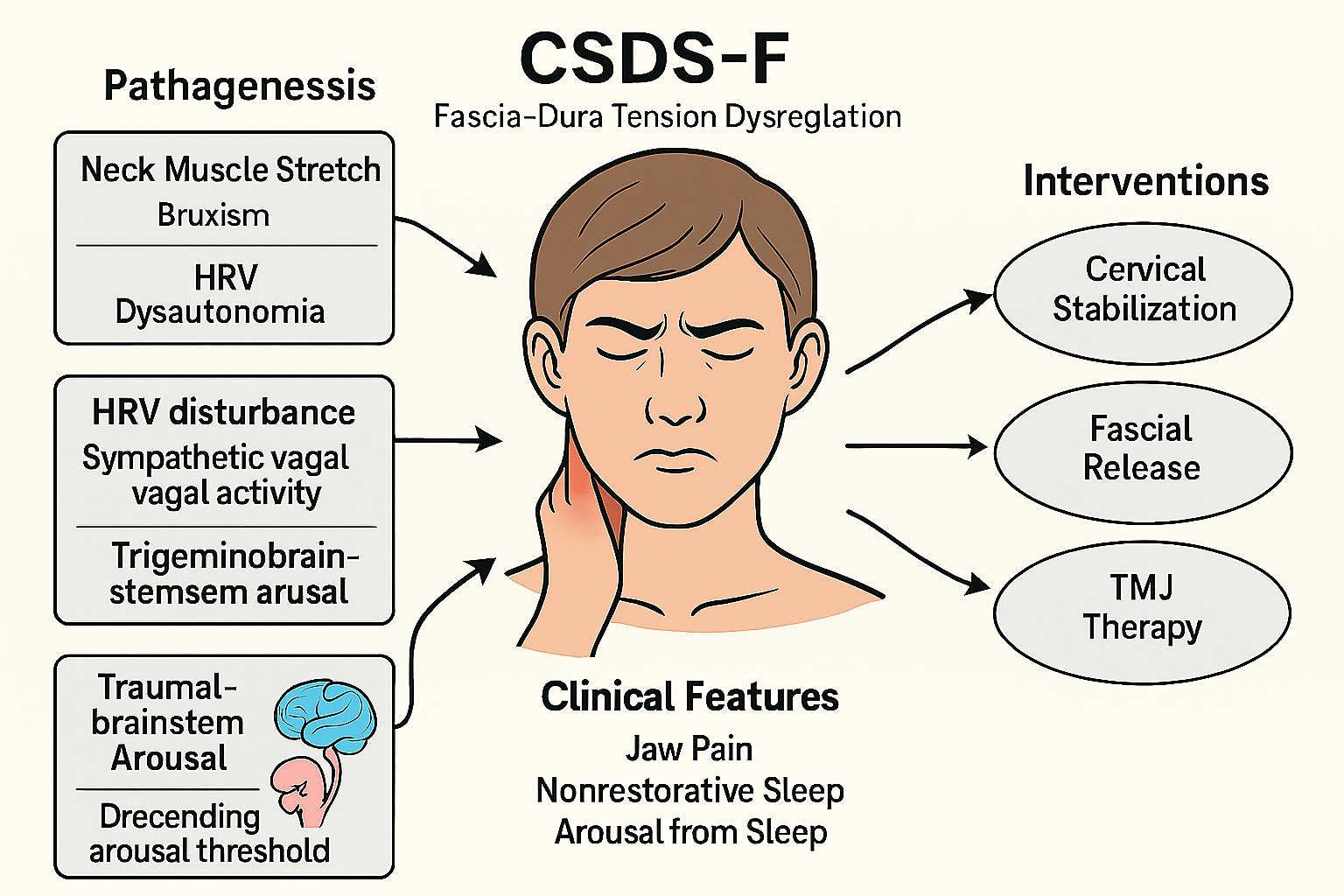
Figure 6:
Schematic diagram of CSDS-F type (Temporomandibular Disorder-Driven Cervicogenic Sleep Disorder Syndrome).
This subtype is characterized by fascial-dural tension dysregulation secondary to temporomandibular joint (TMJ) dysfunction, bruxism, and neck muscle strain. The cascade of HRV-related dysautonomia and trigeminobrainstem activation may lower the arousal threshold and lead to sleep disruption. Multimodal intervention includes cervical stabilization, fascial release, and TMJ therapy.
Abbreviations and Explanations:
CSDS-F:Cervicogenic Sleep Disorder Syndrome - Type F; TMJ: Temporomandibular Joint; HRV: Heart Rate Variability - a marker of autonomic nervous system flexibility; Dysautonomia: Autonomic nervous system imbalance or dysfunction; Trigemino-brainstem arousal: Activation of brainstem circuits via trigeminal input; Cervical Stabilization: Manual or rehabilitative techniques for stabilizing the cervical spine; Fascial Release: Manual therapy to reduce abnormal fascial tension
The CSDS-F subtype represents a crossover mechanism in which cervical fascial imbalances impact the functionality of the temporomandibular region. Anatomical continuity exists between the deep cervical fascia and the myofascial structures of the temporomandibular complex, particularly at the intersection of the sternocleidomastoid, pterygoid, and temporalis fasciae. Abnormal tension in these regions may propagate through fascial pathways to influence masticatory muscle function, thereby initiating or exacerbating temporomandibular disorder (TMD) [100]. Clinical observations suggest that TMD patients frequently present with restricted neck mobility and abnormal posture, both of which may amplify TMD symptom expression and further disrupt sleep architecture [101].
Key Pathophysiological Mechanisms:
Cervical fascial tension and sleep bruxism : Sleep bruxism is a commonly observed comorbidity in TMD and is closely associated with heightened sympathetic activity, reduced vagal tone, and abnormal muscle electrical activity [102]. Instability or abnormal tone in deep cervical stabilizers is considered a potential trigger. Prospective trials have demonstrated that cervical function training improves sleep quality and reduces morning muscle tightness and fatigue in TMD patients with cervical instability [103].
Autonomic dysregulation and sympathetic overactivity : TMD may be accompanied by dysregulation of the autonomic nervous system (ANS), often manifesting as increased sympathetic and decreased parasympathetic activity [102,103]. Although the low-frequency/high-frequency (LF/HF) ratio is frequently used as a marker of sympathovagal balance, its interpretability remains debated. Central inhibition deficits under chronic pain conditions may elevate sympathetic-driven arousals, further deteriorating sleep quality [104,105].
Trigeminal-brainstem-arousal system axis : Chronic nociceptive input originating from TMD can activate the ascending reticular activating system (ARAS) via the trigeminal nerve, particularly impacting locus coeruleus (LC) discharge patterns. This modulation affects norepinephrine dynamics, leading to lowered arousal thresholds and increased micro-arousal frequency, thereby fragmenting sleep architecture [106,107].
Bidirectional amplification of pain and sleep disturbance : Some evidence suggests that subjective deterioration in sleep quality and increased nocturnal arousals may precede overt TMD pain symptoms, supporting the notion that sleep disturbance may act as a prodromal feature in the TMD pain phenotype. This points to a self-reinforcing pathological loop involving masticatory musculature, trigeminal afferents, and brainstem arousal circuits [108,109].
Myodural bridge (MDB) tension disrupting cerebrospinal fluid (CSF) dynamics : Deep cervical muscles, including the rectus capitis posterior minor, major, and obliquus capitis inferior, form anatomical connections with the intracranial dura via the MDB [58,86-88,93]. Structural studies indicate that this linkage contributes to both proprioception and CSF flow regulation. Alterations in the morphology or tension of the MDB may disturb subarachnoid pressure dynamics and disrupt CSF tidal movement, thereby impairing glymphatic clearance during deep sleep phases [110,111].
In summary, CSDS-F embodies a fascial-neuromuscular–cerebral crossover pathway, in which cervical fascial tension contributes to TMD, which in turn promotes nocturnal arousals and non-restorative sleep. These findings underscore the necessity of addressing the bidirectional interaction between cervical myofascial balance and temporomandibular function in sleep disorder interventions.
CSDS-G type
Occipito-Atlanto-Axial Instability (OAAI) (Figure 7)
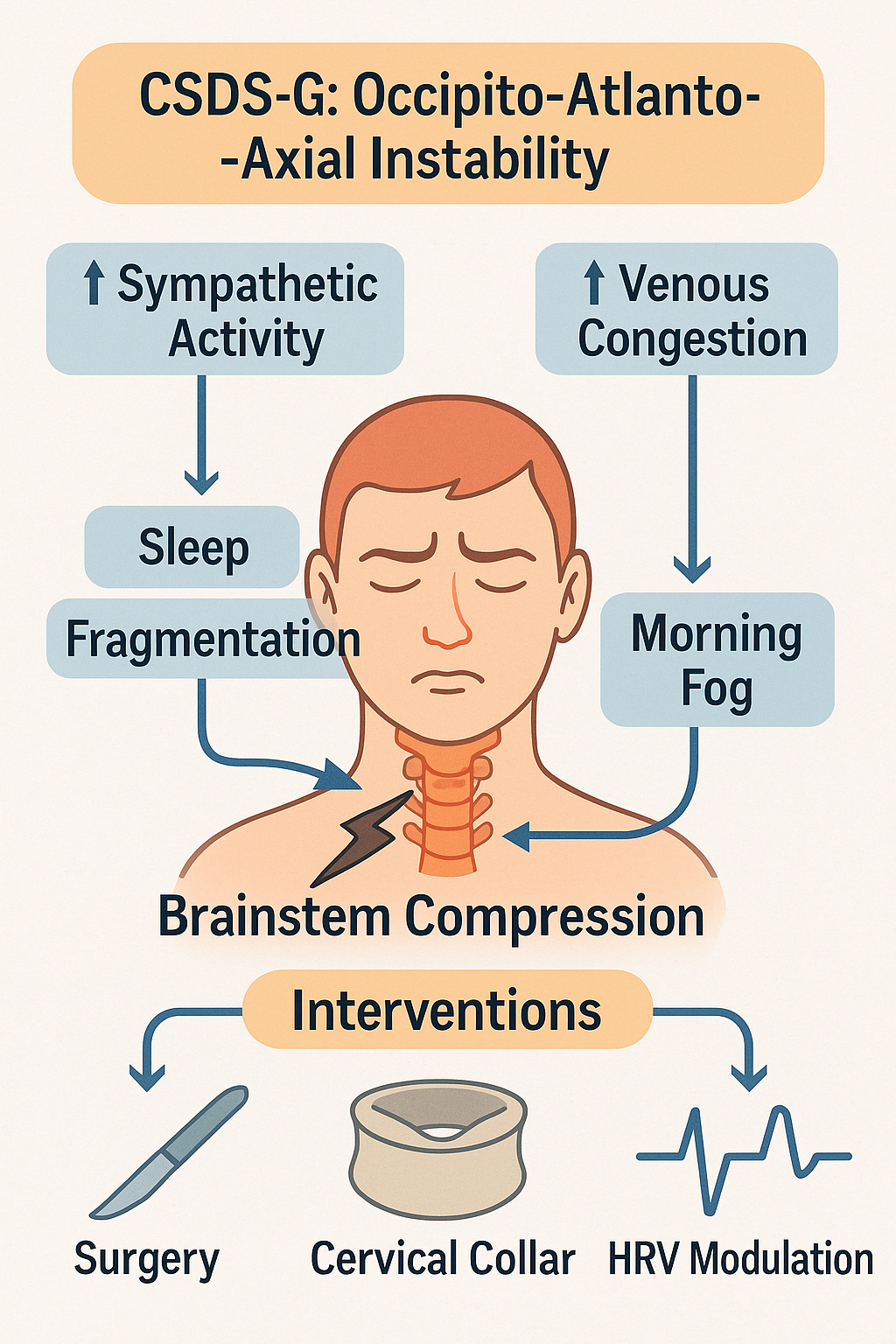
Figure 7:
Schematic diagram of CSDS-G type (Occipito-Atlanto-Axial Instability subtype of Cervicogenic Sleep Disorder Syndrome).
Instability of the occipito-atlanto-axial complex may result in mechanical brainstem compression, increasing sympathetic tone and venous congestion. These factors contribute to sleep fragmentation and cognitive symptoms like “morning fog.” Clinical interventions include cervical stabilization via collar, surgical fixation, and autonomic modulation strategies such as heart rate variability (HRV)-based therapies.
Abbreviations and Explanations:
CSDS-G:Cervicogenic Sleep Disorder Syndrome - Type G; OAAI: Occipito-Atlanto-Axial Instability; HRV: Heart Rate Variability; HRV Modulation: Autonomic regulation through interventions targeting heart rate variability; Cervical Collar: Orthotic device for cervical immobilization
The Cervicogenic Sleep Disorder Syndrome-G subtype (CSDS-G), known as Occipito-Atlanto-Axial Instability (OAAI), refers to sleep disturbances associated with brainstem compression resulting from structural instability in the occiput–atlas–axis complex (C0-C2). It emphasizes how upper cervical anatomical abnormalities disrupt the central arousal system. The hallmark of this subtype is instability of the upper cervical spine, particularly atlantoaxial joint (C1-C2) hypermobility, subluxation, or rotational malalignment [112-118]., leading to anterior medullary compression and subsequent dysregulation of autonomic function, cerebrospinal fluid (CSF) dynamics, and sleep rhythm.
Core Pathophysiological Mechanisms
Brainstem compression and sleep dysregulation : Patients with upper cervical instability often present with central sleep apnea (CSA) and nocturnal hypersomnolence. These symptoms may result from a decreased arousal threshold and heightened sympathetic tone [112-114]. Structural deformation can directly compress the medulla oblongata-especially its ventrolateral region-disrupting the function of the Pre-Bötzinger complex and ascending reticular activating system (ARAS), thereby impairing arousal control and sleep-breathing regulation [115,116].
Imaging findings typically reveal an increased atlantodental interval (ADI) ≥ 3 mm or reduced posterior atlantodental interval (PADI) < 13 mm, indicative of atlantoaxial instability [117]. These spinal abnormalities often correlate with polysomnography (PSG) findings of reduced slow-wave sleep (SWS) and increased nocturnal awakenings, supporting the “structural lesion–arousal dysfunction–sleep fragmentation” cascade.
Venous outflow obstruction and csf circulatory imbalance : Rotational deformities at C1-C2 or cranial base anomalies (e.g., basilar invagination) may compress venous outflow tracts, including the sigmoid sinus and jugular bulb, leading to elevated transverse sinus pressure gradients and impaired CSF drainage [118]. This disruption may impair glymphatic clearance, inhibit N3 sleep generation, exacerbate metabolite accumulation, and manifest as morning brain fog.
CSDS-G illustrates a closed-loop mechanism linking “structural compression–brainstem arousal chain–sympathetic activation–circadian rhythm disturbance.” Clinical evaluation should integrate imaging modalities (e.g., MRI and CT measuring ADI/PADI), autonomic function testing (e.g., heart rate variability [HRV] analysis), and PSG for early identification and subtype classification. Interventions may include neural decompression surgery, positional therapy, cervical bracing, and HRV modulation, establishing physiological phenotypes for AI-assisted individualized modeling.
CSDS-H type
Cerebro-venolymphatic Outflow Disorder (Figure 8)
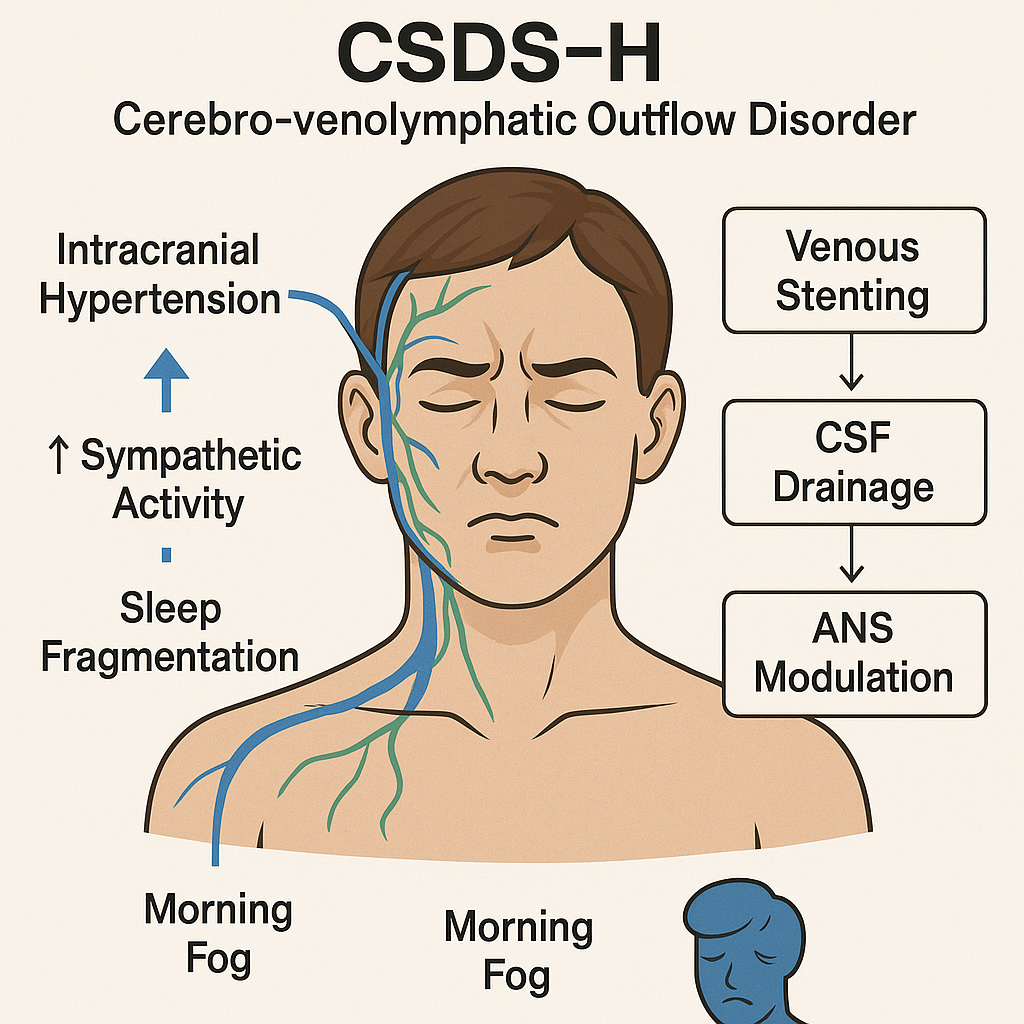
Figure 8:
Schematic diagram of CSDS-H type (Cerebro-venolymphatic Outflow Disorder subtype of Cervicogenic Sleep Disorder Syndrome).
In this subtype, disruption of cerebrospinal and venolymphatic outflow leads to intracranial hypertension, which is often exacerbated by sympathetic overactivation and sleep fragmentation. Clinical symptoms frequently include cognitive clouding and “morning fog.” Therapeutic approaches may involve venous stenting, cerebrospinal fluid (CSF) drainage procedures, and modulation of the autonomic nervous system (ANS) to restore physiological outflow and relieve pressure-related symptoms.
Abbreviations and Explanations:
CSDS-H stands for Cervicogenic Sleep Disorder Syndrome - Type H; CSF: Cerebrospinal Fluid, the clear fluid surrounding the brain and spinal cord, essential for cushioning and waste removal; ANS: Autonomic Nervous System, which regulates involuntary physiological functions including vascular tone and lymphatic drainage. Venous Stenting involves the placement of a stent in cerebral venous sinuses to improve drainage and reduce intracranial pressure; CSF Drainage: Therapeutic removal of excess cerebrospinal fluid to alleviate elevated intracranial pressure; ANS Modulation: Interventions aimed at balancing sympathetic and parasympathetic nervous activity, often through neurostimulation, breathing techniques, or pharmacologic agents
The CSDS-H subtype (cranial venous outflow-related sleep disorder) reflects a pathological cascade of “venous congestion → cerebrospinal fluid (CSF) dysregulation → slow-wave suppression and sympathetic activation.” Intracranial hypertension due to dural venous sinus stenosis can trigger dural stretch reflexes, heightening sympathetic tone and subsequently disrupting autonomic nervous system (ANS) regulation.
Key mechanisms of CSDS-H include:
Elevated venous sinus pressure gradient : Congenital stenosis of the transverse or sigmoid sinus, or structural compressions from atlantoaxial rotation or elongated styloid processes, may elevate intracranial venous pressure and inhibit CSF resorption by arachnoid granulations [119]. Such venous blockage interferes with glymphatic clearance, especially during N3-stage slow-wave sleep, contributing to sleep fragmentation and sympathetic arousal [120,121].
CSF dynamics demonstrate strong synchronization with low-frequency EEG waves during sleep, indicating neuro-fluid coupling [120]. MRI reveals rhythmic intracranial pressure fluctuations during the night, with abnormal waveforms associated with reduced slow-wave activity [122].
Venous sinus stenting (VSS) can relieve symptoms linked to elevated intracranial pressure by lowering pressure gradients across the transverse or sigmoid sinus. Studies have shown a strong correlation between lumbar opening pressure (LOP) and venous sinus gradients, supporting the mechanistic basis for VSS in intracranial pressure modulation [123,124]. Though its effect on N3% or apnea–hypopnea index (AHI) remains inconclusive, VSS has been reported to improve symptoms like headache and tinnitus, thereby indirectly enhancing sleep quality [125].
Suppression of slow-wave activity and impaired glymphatic clearance : Impaired venous return (e.g., transverse sinus stenosis) may disrupt CSF flow and elevate intracranial pressure, weakening the glymphatic system’s ability to remove neurotoxins. This system, driven by arterial pulsatility, relies on CSF inflow through periarterial spaces to mix with interstitial fluid (ISF), facilitating waste clearance. Its efficiency peaks during non-rapid eye movement (NREM) sleep, particularly during N3-stage slow-wave sleep [126,127].
Ringstad and Eide [128] demonstrated, via MRI contrast tracing, that CSF outflow primarily occurs through the dura near the superior sagittal sinus and is limited by venous resistance. In idiopathic intracranial hypertension (IIH), the degree of transverse sinus stenosis is strongly associated with glymphatic dysfunction, implicating venous impairment in CSF-ISF exchange failure, toxic metabolite accumulation, and “morning brain fog” [129].
Animal studies further link enhanced delta power with increased CSF perfusion, offering a physiological rationale for the inverse relationship between sleep depth and neurotoxic burden [130].
Autonomic dysregulation : Elevated intracranial pressure can stretch the dura and activate trigeminal sensory afferents, enhancing sympathetic output and disturbing ANS homeostasis [131]. Increased sympathetic tone is considered a primary driver of reduced heart rate variability (HRV), particularly during nocturnal sleep [132]. Low HRV not only reflects parasympathetic suppression but also correlates with elevated inflammatory markers such as IL-6 and higher arousal rates, suggesting a role for ANS dysfunction as a central bridge between disrupted sleep architecture and systemic inflammation [133].
Therefore, CSDS-H highlights the importance of multidimensional assessment strategies, integrating imaging-based venous/lymphatic flow evaluation with polysomnography (PSG) and HRV analysis, to enable individualized diagnosis and AI-assisted modeling of cervicogenic sleep disorders.
CSDS-I type
Neurovascular Compression-Related Type (Figure 9)
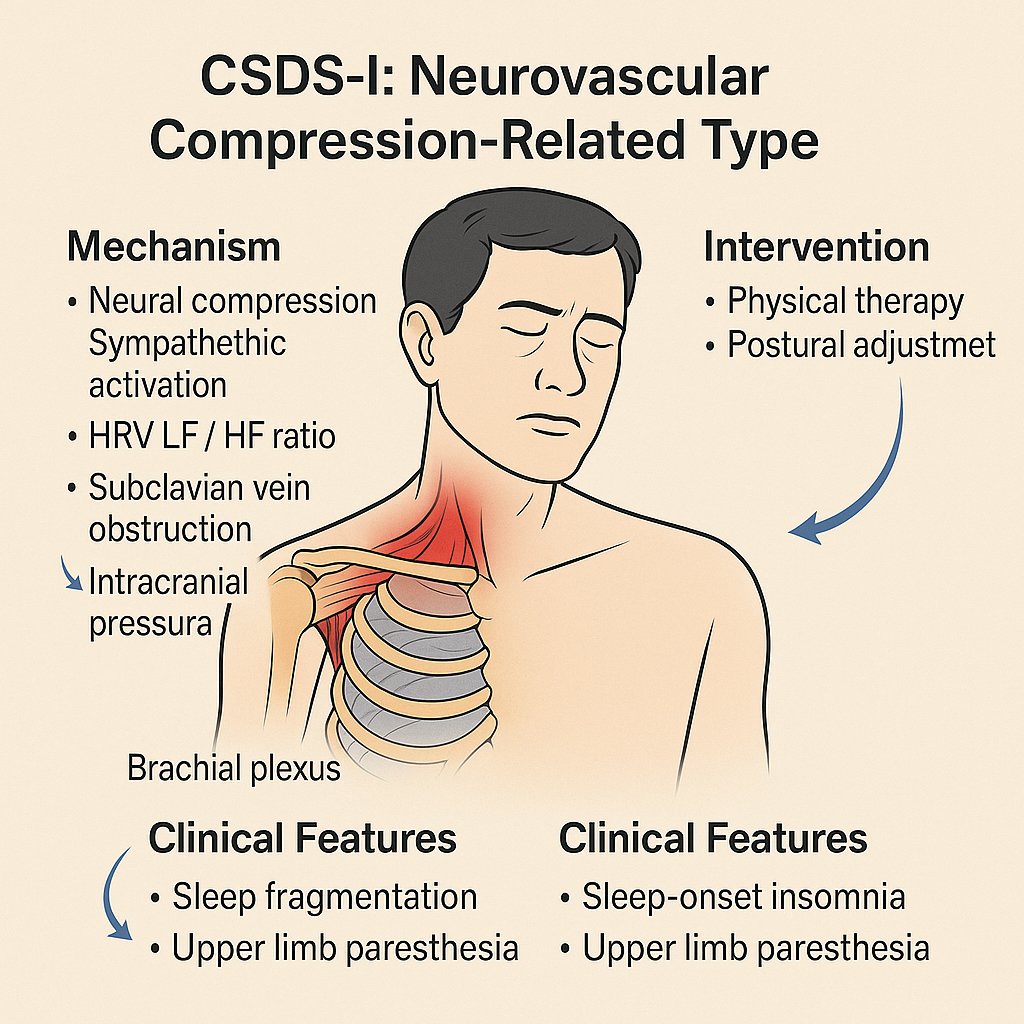
Figure 9:
Schematic diagram of CSDS-I type (Neurovascular Compression-Related Subtype of Cervicogenic Sleep Disorder Syndrome).
This subtype is characterized by compression of the brachial plexus and subclavian vasculature, typically due to thoracic outlet dysfunction. Mechanistically, neural compression leads to sympathetic overactivity, altered autonomic balance (HRV changes), and impaired venous return, potentially contributing to increased intracranial pressure. Clinical manifestations include sleep fragmentation, sleep-onset insomnia, and upper limb paresthesia. Management strategies may involve physical therapy and postural realignment.
Abbreviations and Explanations:
CSDS-I: Cervicogenic Sleep Disorder Syndrome - Type I (Neurovascular Compression-Related); HRV: Heart Rate Variability, an index reflecting autonomic nervous system balance; LF / HF ratio: Low Frequency to High Frequency Ratio in HRV analysis; often elevated in sympathetic overactivation; Brachial plexus: A complex network of nerves originating from the cervical spinal cord and innervating the upper limb; Subclavian vein: A major vein involved in venous return from the upper limb and brain obstruction can increase cerebral venous pressure; Intracranial pressure: The pressure within the skull, which may rise secondary to impaired venous outflow.
The Cervicogenic Sleep Disorder Syndrome-I (CSDS-I) subtype is based on Thoracic Outlet Syndrome (TOS), which involves anatomical narrowing in the subclavian region-such as the scalene triangle, costoclavicular space, or sub-pectoral minor space-leading to intermittent compression of the brachial plexus, subclavian vein, and sympathetic trunk [134-138]. This compression may trigger sympathetic activation and impair venous return, resulting in autonomic dysregulation and disruption of sleep architecture. The underlying pathophysiology can be summarized as: "neurovascular compression → HRV disruption + CSF dynamic imbalance → sleep fragmentation."
Key mechanisms in CSDS-I include:
Neural compression and sympathetic activation : Supine positioning or arm abduction during sleep may stretch the brachial plexus, triggering sympathetic reflexes. This results in increased low-frequency to high-frequency (LF/HF) ratio in heart rate variability (HRV) [134], prolonged sleep latency, and frequent alpha wave-related arousals [135].
Subclavian venous obstruction and CSF tidal imbalance : Compression of the subclavian vein can hinder intracranial venous drainage, subsequently affecting cerebrospinal fluid (CSF) reabsorption. This pathomechanism is recognized clinically as Venous Thoracic Outlet Syndrome (VTOS), where venous obstruction between the clavicle and first rib causes impaired blood flow, thrombosis, and increased intracranial pressure, thereby inhibiting arachnoid granule-mediated CSF reabsorption [136]. Additionally, body positioning significantly influences intracranial fluid dynamics; the supine posture may elevate intracranial venous pressure [137], further disrupting CSF circulation and clearance.
Brachial plexus nociception and arousal chain activation : Chronic pain stimuli from brachial plexus compression may be amplified by the arousal system during N2-N3 non-rapid eye movement sleep, leading to micro-arousals and fragmented sleep. Patients frequently report awakening due to hand numbness or traction pain during turning movements, along with morning brain fog and non-restorative sleep. Studies indicate that approximately 69.5% of TOS patients present with Pittsburgh Sleep Quality Index (PSQI) ≥ 5, and the severity of upper limb dysfunction (measured by DASH score) correlates with sleep quality (ρ = 0.58) [138].
The neurovascular imbalance associated with TOS is incorporated into CSDS-I, reinforcing the integrative “cervical-shoulder-thoracic outlet-brain” vertical pathway model. Evaluation of CSDS-I emphasizes parallel quantification using dynamic imaging modalities (e.g., MRI and ultrasound) and functional sleep tools (e.g., PSQI and DASH). Treatment follows a stepwise approach of “conservative → blockade → outlet decompression,” beginning with physical therapy and postural correction, progressing to nerve blocks when necessary, and ultimately considering surgical decompression.
CSDS-J subtype
System-Reactive CSDS (Figure 10)
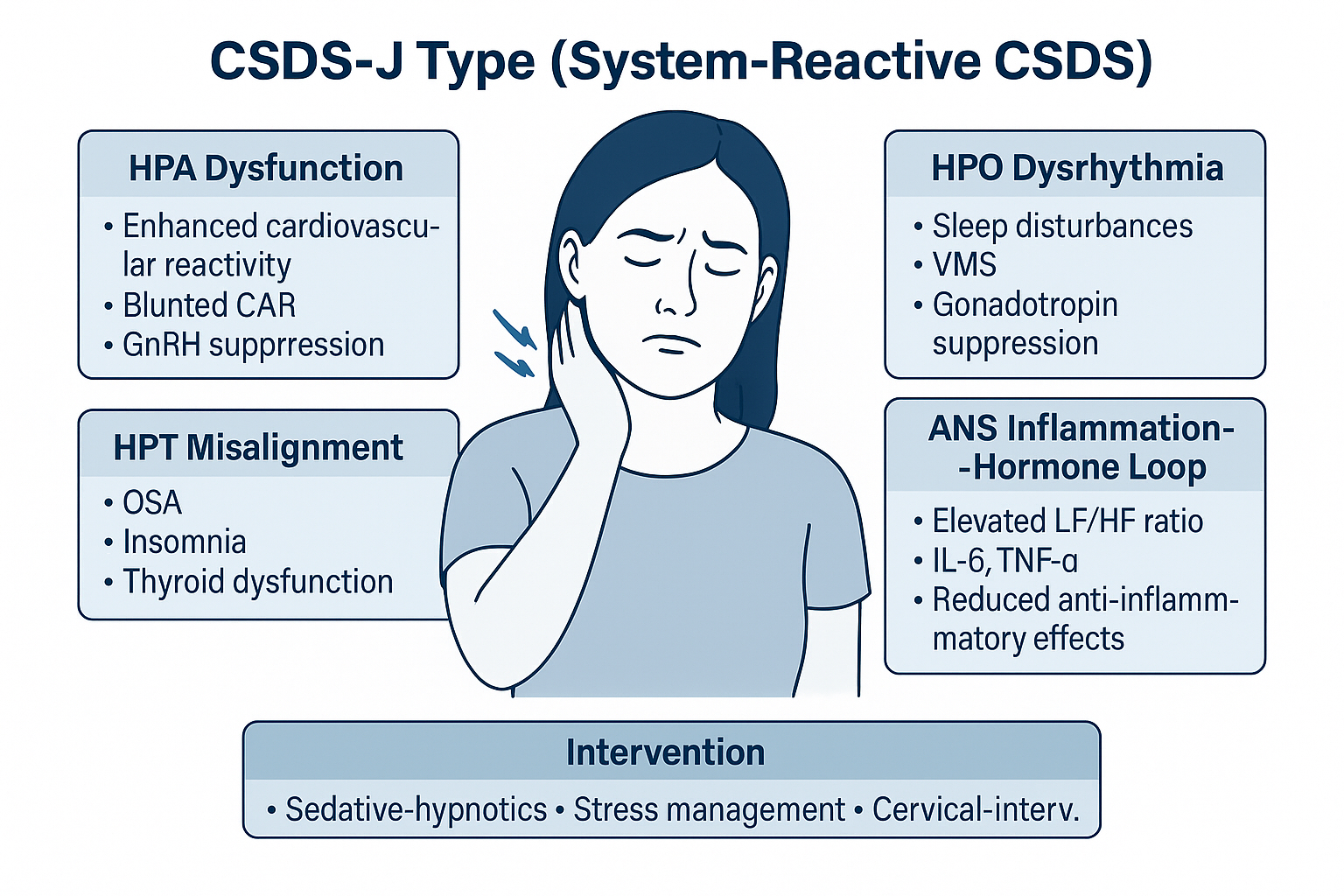
Figure 10:
Schematic diagram of CSDS-J type (System-Reactive Subtype of Cervicogenic Sleep Disorder Syndrome).
This subtype represents a complex interaction between multiple neuroendocrine axes and autonomic-inflammatory feedback loops, resulting in persistent sleep disturbances. Dysregulations across the HPA (hypothalamic-pituitary-adrenal), HPO (hypothalamic-pituitary-ovarian), and HPT (hypothalamic-pituitary-thyroid) axes interact with autonomic dysfunction and inflammatory mediators. Clinically, patients may present with insomnia, vasomotor instability, and hormonal imbalance. Intervention targets include sedative-hypnotic therapy, stress response modulation, and cervical-related peripheral interventions.
Abbreviations and Explanations:
CSDS-J: Cervicogenic Sleep Disorder Syndrome - Type J (System-Reactive Subtype); HPA: Hypothalamic-Pituitary-Adrenal Axis, responsible for stress and cortisol regulation; CAR: Cortisol Awakening Response, a key biomarker of HPA axis function; GnRH: Gonadotropin-Releasing Hormone, whose suppression reflects hypothalamic-pituitary dysfunction; HPT: Hypothalamic-Pituitary-Thyroid Axis, involved in metabolic and sleep regulation; OSA: Obstructive Sleep Apnea, contributing to disrupted sleep architecture; HPO: Hypothalamic-Pituitary-Ovarian Axis, relevant in reproductive and vasomotor regulation; VMS: Vasomotor Symptoms, such as hot flashes and night sweats, linked to hormonal shifts; ANS: Autonomic Nervous System, particularly when imbalanced toward sympathetic dominance; IL-6: Interleukin-6, a pro-inflammatory cytokine elevated in stress and systemic inflammation; TNF-α: Tumor Necrosis Factor-alpha, another inflammatory mediator associated with sleep disruption; LF/HF Ratio: Low Frequency to High Frequency ratio, a heart rate variability (HRV) index reflecting sympathetic-parasympathetic balance; Cervical-Interv.: Cervical intervention, referring to physical therapies targeting cervical somatic dysfunctions.
The CSDS-J subtype, or System-Reactive Cervicogenic Sleep Disorder Syndrome (CSDS), refers to a dysregulation of the hypothalamic-pituitary-target gland axes—including the HPA, HPT and HPO axes-and the ANS, triggered by the coexistence of chronic neck pain and persistent sleep deprivation. This subtype is often accompanied by systemic inflammatory activation and multi-axis disruption across sleep, endocrine, and immune regulation.
Core mechanisms of CSDS-J include:
HPA axis dysregulation : Patients with chronic cervical pain frequently exhibit enhanced cardiovascular reactivity and a diminished cortisol awakening response (CAR) under acute psychological stress, suggesting HPA dysfunction that is tightly linked with sympathetic activation within the ANS [139,140]. Similarly, patients with cervicogenic headache show aberrant cortisol response patterns to mild cognitive stimulation, highlighting the vulnerability of the HPA axis in chronic pain and arousal regulation [141]. Chronic activation of the HPA axis may inhibit the release of gonadotropin-releasing hormone (GnRH) and thyroid-stimulating hormone (TSH), disrupting circadian patterns in the reproductive and thyroid axes and impeding normal nocturnal hormone surges during deep sleep (N3) [142,143]. These neuroendocrine imbalances are strongly implicated in non-restorative sleep, morning fatigue, and disordered sleep architecture in CSDS.
HPT axis misalignment : Thyroid hormone irregularities can significantly impact sleep regulation. In primary hypothyroidism, the incidence of obstructive sleep apnea syndrome (OSAS) is elevated, possibly due to mucopolysaccharide accumulation causing airway collapse and respiratory muscle weakness. Following levothyroxine treatment, patients with hypothyroidism showed marked reductions in apnea-hypopnea index (AHI), suggesting a therapeutic role in sleep-related breathing disorders [144]. Conversely, hyperthyroidism is associated with insomnia symptoms including difficulty initiating sleep, frequent nocturnal awakenings, and reduced sleep efficiency. These are primarily driven by heightened sympathetic tone and metabolic overactivation, both of which impair the normal functioning of the sleep-wake system. Antithyroid treatment has been reported to improve sleep onset latency and REM sleep architecture in most cases [145].
HPO axis compression and menopause-associated CSDS-J : In perimenopausal women, hormonal fluctuations in estrogen and progesterone are commonly associated with disturbed sleep architecture, characterized by sleep onset difficulties, frequent awakenings, and early morning arousals [146]. Vasomotor symptoms (VMS), such as hot flashes and nocturnal sweating, lower the arousal threshold, suggesting that menopausal insomnia may involve bi-directional coupling between the HPO axis and central sympathetic networks. Chronic cervical tension and heightened sympathetic activity may further exacerbate HPA axis activation, thereby disturbing HPO rhythmicity [147]. Studies have shown that patients with chronic neck pain demonstrate enhanced cardiovascular reactivity and altered CAR under psychological stress-markers of sympathetic-HPA dysregulation. Additional findings reveal that chronic stress and elevated cortisol suppress GnRH secretion, leading to reduced luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels, resulting in HPO axis compression and reproductive hormonal imbalance [148]. Taken together, menopause-associated CSDS-J may reflect a neuroendocrine imbalance involving cervical-origin sympathetic overactivation, heightened HPA activity, and HPO suppression, forming a "cervical-stress-hormone-sleep" interference loop.
ANS as a hub for inflammation-hormone feedback : Enhanced nocturnal sympathetic activity, reflected by an increased low-frequency/high-frequency (LF/HF) ratio in heart rate variability (HRV), may inhibit parasympathetic anti-inflammatory pathways and elevate circulating pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) [149]. These cytokines can in turn activate the HPA axis, forming a positive feedback loop between inflammation and hormone dysregulation [150].
In summary, the CSDS-J subtype illustrates how cervical nociceptive input acts as a chronic stressor that disrupts neuroendocrine and immune homeostasis. Therapeutic strategies should integrate sedative-hypnotic agents, stress management protocols, and interventions targeting cervical spine disorders (Table 1).
Table 1: Summary of the 10 CSDS subtypes (A-J).
|
Subtype (A-J) |
Core Pathophysiology |
Clinical Features (Physical & Auxiliary Exams) |
Diagnostic Clues (Etiology, History, Epidemiology) |
Differential Diagnosis (Intra-CSDS & Others) |
|
CSDS-A |
Elevated arousal, short sleep duration, poor SWS, HRV metrics [71] |
Chronic neck pain, cervical spondylosis history, PSG & HRV changes [5,6,73] |
Primary insomnia, anxiety-related insomnia, vs. CSDS-C (pain) [72] |
|
|
CSDS-B |
Vagal suppression with circadian misalignment [74-76] |
Delayed sleep phase, daytime hypersomnolence, melatonin shift [74] |
History of circadian rhythm disorder, HRV vagal deficit, actigraphy [75] |
DSPS, idiopathic insomnia vs. CSDS-A (autonomic hyperarousal) [76] |
|
CSDS-C |
Pain-induced cortical arousal via nociceptive pathway [77-80] |
Frequent nocturnal awakening, reduced N3, pain complaints [78] |
Cervical pain > 3 months, MRI spondylosis, twin studies [79] |
Depression-related insomnia, fibromyalgia, vs. CSDS-A [80] |
|
CSDS-D |
Cervical dystonia-induced thalamocortical disturbance [81-85] |
Muscle tone asymmetry, prolonged sleep latency, PSG N3↓ [83] |
Known dystonia, botox response, EMG abnormalities [82,84] |
Parkinsonism, primary dystonia vs. CSDS-G (brainstem) [85] |
|
CSDS-E |
Myodural bridge tension disrupting CSF dynamics [86-88] |
Morning brain fog, abnormal HRV, reduced SWS, cranial pressure [89-92] |
Head posture anomalies, neck stiffness, C0-C2 imaging [88,93] |
Idiopathic intracranial hypertension, tension-type headache [24] |
|
CSDS-F |
TMD linked via fascial continuity & sympathetic loop [100-105] |
Sleep bruxism, jaw pain, cervical restriction, HRV shift [102-103] |
TMJ dysfunction, neck-TMD co-existence, dental evaluation [101] |
Primary TMD, dental-origin bruxism vs. CSDS-A (autonomic) [104] |
|
CSDS-G |
Occipito-atlanto-axial instability compressing brainstem [112-118] |
Central sleep apnea, SWS loss, high ADI/PADI [113-114] |
C1-C2 instability, os odontoideum, CT/MRI correlation [117] |
Chiari malformation, congenital instability vs. CSDS-D [115] |
|
CSDS-H |
Cerebro-venolymphatic outflow disorder → ICP ↑ [119-129] |
Venous congestion, N3↓, low HRV, VSS response [123-125] |
Sinus stenosis, jugular reflux, contrast MRI [124,128] |
IIH, migraine, vs. CSDS-G (mechanical compression) [129-130] |
|
CSDS-I |
Thoracic outlet neurovascular compression [134-138] |
Arm numbness, sleep-position arousals, HRV alteration [134] |
Positive DASH score, positional imaging, TOS testing [135-136] |
Peripheral neuropathy, obstructive apnea vs. CSDS-J [137-138] |
|
CSDS-J |
HPA/HPT/HPO axis + ANS dysregulation [139-150] |
Systemic inflammation, hormone shifts, fatigue, poor sleep [141-143] |
Chronic stress, menopause, hormone labs, IL-6↑ [146-148] |
Endocrine disorders, chronic fatigue vs. CSDS-B/H [149-150] |
Abbreviations: LF/HF: Low-frequency to high-frequency ratio (HRV index); HF-HRV: High-frequency heart rate variability; SWS: Slow-wave sleep (N3 stage); PSG: Polysomnography; TMD: Temporomandibular disorder; CSF: Cerebrospinal fluid; ICP: Intracranial pressure; ADI/PADI: Atlanto-dental interval / Posterior ADI; VSS: Venous sinus stenting; DASH: Disabilities of the Arm, Shoulder, and Hand index; IL-6: Interleukin 6, inflammatory biomarker
Conclusion and Future Perspectives
Innovative proposal and foundational framework
Cervicogenic Sleep Disorder Syndrome (CSDS), as a newly proposed disease spectrum, highlights the pivotal role of both structural and functional cervical abnormalities in the pathogenesis of sleep disorders. Through a systematic mechanistic analysis, this study establishes a CSDS subtype classification system based on structural, functional, and circadian disruptions. This provides a foundational framework for future clinical diagnosis, mechanistic exploration, and precision intervention.
Four core characteristics
Pathogenicity, Systemicity, Identifiability, and Intervenability
Pathogenicity : CSDS emphasizes cervical dysfunction as a direct etiological trigger. Its identification can be quantified through imaging modalities, ultrasound, and three-dimensional biomechanical analysis [151].
Systemicity : CSDS spans across multiple systems, including the nervous, vascular, immune, and endocrine axes, challenging the conventional brain-centric model of insomnia. It emphasizes how cervical anomalies interfere with the sleep-wake regulation loop via the spinal cord-brainstem-thalamus-cortex-rhythm network coupling. The integration of artificial intelligence and radiomics [152] is accelerating a comprehensive understanding of the cervical-sleep system coupling.
Identifiability : The pathological pathways of CSDS are verifiable. Future research should focus on: establishing a CSDS-specific biomarker recognition system, centered on multimodal imaging, physiological parameters, and biochemical markers [153]; building deep learning-driven automated CSDS subtype classification models [154/a>]; clarifying causal links between cervical dysfunction and distinct sleep disorder phenotypes, thus facilitating mechanistic standardization and international consensus [155].
Intervenability : From a therapeutic standpoint, CSDS has clearly defined intervention targets. Subtype-based individualized strategies, including minimally invasive decompression, myofascial modulation, neuromodulation, and digital therapeutics, may overcome conventional pharmacological dependence in insomnia management [156]. Additionally, early screening and postural correction among high-risk populations (e.g., sedentary workers) may interrupt the pathological cycle of 'cervical degeneration-pain-insomnia-sympathetic hyperactivity-chronic disease risk' [157].
Summary and future directions
CSDS is not a narrowly defined secondary insomnia nor a single anatomical disorder. Instead, it represents a complex multisystem dysregulation syndrome driven by coupled dysfunction. This framework redefines the role of cervical pathology in sleep medicine and establishes a theoretical anchor for precise diagnosis, mechanistic subtyping, and interdisciplinary intervention.
Future developments should include: Defining diagnostic criteria and evaluation metrics for each subtype; promoting multicenter mechanistic validation; constructing AI-assisted decision-making systems; and developing targeted therapeutic strategies.
The introduction of CSDS represents a paradigm shift toward systems-level precision medicine, offering substantial academic value and clinical potential.
Funding
National Natural Science Foundation of China (Project Approval Number: 81200858); Jiangsu Province 333 High-level Talent Training Project [Certificate No.: (2022) No. 3-10-007].
Clinical Trials from Nanjing Drum Tower Hospital, Affiliated Hospital of Medical School, Nanjing University, the Huai'an Matching Assistance Special Project (2024-2025).
Conflict of Interest
None.
Presentation
None Declared.
References
- Van Someren EJW. Brain mechanisms of insomnia: new perspectives on causes and consequences. Physiol Rev. 2021;101(3):995–1046.
- Fanielle J. Sleep disorders in connection with somatic symptom disorders. Rev Med Liege. 2023;78(56):296298.
- Uhlig BL, Sand T, Nilsen TI, et al. Insomnia and risk of chronic musculoskeletal complaints: longitudinal data from the HUNT study, Norway. BMC Musculoskelet Disord. 2018;19(1):128.
- Van Looveren E, Bilterys T, Munneke W, et al. The association between sleep and chronic spinal pain: a systematic review from the last decade. J Clin Med. 2021;10(17):3836.
- Bisson EF, Mummaneni PV, Michalopoulos GD, et al. Sleep disturbances in cervical spondylotic myelopathy: prevalence and postoperative outcomes—an analysis from the Quality Outcomes Database. Clin Spine Surg. 2023;36(3):112–119.
- Deng Z, et al. Evaluation of sleep quality in patients with cervical spondylosis and analysis of influencing factors. Front Surg. 2023;10:1048780.
- Kim JH, Kim JH, Kim TH. Prevalence of sleep disturbance and its risk factors in patients who undergo surgical treatment for degenerative spinal disease: a nationwide study of 106,837 patients. J Clin Med. 2022;11(19):5932.
- Yang TH, Xirasagar S, Cheng YF, et al. Association of cervical spondylosis with obstructive sleep apnea. Sleep Med. 2020;71:54–58.
- Bisson EF, Mummaneni PV, Michalopoulos GD, et al. Sleep disturbances in cervical spondylotic myelopathy: prevalence and postoperative outcomes—an analysis from the Quality Outcomes Database. Spine (Phila Pa 1976). 2023;48(5):369–376.
- Lee MK, Oh J. The relationship between sleep quality, neck pain, shoulder pain and disability, physical activity, and health perception among middle-aged women: a cross-sectional study. BMC Womens Health. 2022;22(1):186.
- Williams DA, et al. Chronic neck pain and insomnia: evidence of shared etiology in a twin study. Pain Pract. 2021;21(2):203–211.
- Eichenseer SR, Stebbins GT, Comella CL. Beyond a motor disorder: a prospective evaluation of sleep quality in cervical dystonia. Parkinsonism Relat Disord. 2014;20(4):405–408.
- Antelmi E, Ferri R, Provini F, et al. Modulation of the muscle activity during sleep in cervical dystonia. Sleep. 2017;40(7):zsx078.
- Samushiya MA, Ragimova AA, Ivolgin AF, et al. Sleep disorders in patients with cervical dystonia. Zh Nevrol Psikhiatr Im S S Korsakova. 2020;120(12):25–29.
- Ray S, Pal PK, Yadav R. Non-motor symptoms in cervical dystonia: a review. Ann Indian Acad Neurol. 2020;23(4):449–457.
- Kumagai S, Yamaguchi T, Ito M, et al. Vagus nerve stimulation as a modulator of feedforward and feedback neural transmission. arXiv Preprint. 2025;arXiv:2501.18770.
- Vállez García D, Doorduin J, Willemsen ATM, Dierckx RAJO, Otte A. Altered regional cerebral blood flow in chronic whiplash associated disorders. EBioMedicine. 2016;10:249–257.
- Chen Z, Ding Y, Ji X, et al. Long-term safety and efficacy of stenting on correcting internal jugular vein and cerebral venous sinus stenosis. Ann Clin Transl Neurol. 2023;10(8):1305–1313.
- Bai C, Chen Z, Ding Y, et al. Long-term safety and efficacy of stenting on correcting internal jugular vein and cerebral venous sinus stenosis. Ann Clin Transl Neurol. 2023;10(8):1305–1313.
- Bisson EF, Mummaneni PV, Michalopoulos GD, Bydon M, Glassman SD, Foley KT, et al. Sleep disturbances in cervical spondylotic myelopathy: prevalence and postoperative outcomes—an analysis from the Quality Outcomes Database. Clin Spine Surg. 2023;36(3):112–119.
- Hack GD, Koritzer RT, Robinson WL, Hallgren RC, Greenman PE. Anatomic relation between the rectus capitis posterior minor muscle and the dura mater. Spine (Phila Pa 1976). 1995;20(23):2484–2486.
- Chen Z, Ding Y, Ji X, et al. Long-term safety and efficacy of stenting on correcting internal jugular vein and cerebral venous sinus stenosis. Ann Clin Transl Neurol. 2023;10(8):1305–1313.
- Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111.
- Yin J, Huang X, Shrestha S, et al. Venous outflow impairment and glymphatic dysfunction: a multimodal MRI study in idiopathic intracranial hypertension. Neurology. 2023;101(15):e1480–e1491.
- Vallez García D, Doorduin J, Willemsen ATM, de Jong JR, Geuze E, van Berckel BN, et al. Altered regional cerebral blood flow in chronic whiplash associated disorders. EBioMedicine. 2016;10:249–257.
- Rahimizadeh A, Malekmohammadi Z, Karimi M, Kanaan I, Rahimizadeh S. Unstable os odontoideum contributing to cervical myelopathy and obstructive sleep apnea. Surg Neurol Int. 2019;10:125.
- Van Someren EJW. Brain mechanisms of insomnia: new perspectives on causes and consequences. Physiol Rev. 2021;101(3):995–1046.
- Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14(12):1539–1552.
- Shahidi B, Sannes T, Laudenslager M, Maluf KS. Cardiovascular responses to an acute psychological stressor are associated with the cortisol awakening response in individuals with chronic neck pain. Physiol Behav. 2015;150:93–98.
- Williams DP, Koenig J, Carnevali L, et al. Heart rate variability and inflammation: a meta-analysis of human studies. Brain Behav Immun. 2019;80:219–226.
- Sun SY, Chen GH. Treatment of circadian rhythm sleep–wake disorders. Curr Neuropharmacol. 2022;20(6):1022–1034.
- Milenovic N, Klašnja A, Skrbic R, et al. Sleep problems and disabilities of the arm, shoulder, and hand in persons with thoracic outlet syndrome. Int J Environ Res Public Health. 2022;19(19):12486.
- Fanielle J. Sleep disorders in connection with somatic symptom disorders. Rev Med Liege. 2023;78(56):296298.
- Eichenseer SR, Stebbins GT, Comella CL. Beyond a motor disorder: a prospective evaluation of sleep quality in cervical dystonia. Parkinsonism Relat Disord. 2014;20(4):405408.
- Chen Z, Ding Y, Ji X, et al. Longterm safety and efficacy of stenting on correcting internal jugular vein and cerebral venous sinus stenosis. Ann Clin Transl Neurol. 2023;10(8):13051313.
- Sic A, Bogicevic M, Brezic N, Nemr C, Knezevic NN. Chronic stress and headaches: the role of the HPA axis and autonomic nervous system. Biomedicines. 2025;13(2):463.
- Yin Q, Wang JF, Wang S, et al. Thyroid disease-related sleep disorders and its diagnostic and therapeutic recommendations: a literature review. Perioper Precis Med. 2023;1:101–118.
- Mbiydzenyuy NE, Qulu LA. Stress, hypothalamic-pituitary-adrenal axis, hypothalamic-pituitary-gonadal axis, and aggression. Metab Brain Dis. 2024;39:1613–1636.
- Wang L, Huang T, Chen W, et al. Stress-induced hypothalamic–pituitary–gonadal axis dysfunction: implications for female reproductive health. Front Endocrinol (Lausanne). 2023;14:1176453.
- Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258.
- Sateia MJ. International classification of sleep disorders—third edition: highlights and modifications. Chest. 2014;146(5):1387–1394.
- Lee SL, Sena M, Greenholz SK, Fledderman M. A multidisciplinary approach to the development of a cervical spine clearance protocol: process, rationale, and initial results. J Pediatr Surg. 2003;38:358–362.
- Brinjikji W, Luetmer PH, Comstock B, et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR Am J Neuroradiol. 2015;36(4):811–816.
- Miotto R, Wang F, Wang S, Jiang X, Dudley JT. Deep learning for healthcare: review, opportunities and challenges. Brief Bioinform. 2018;19(6):1236–1246.
- Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25(1):44–56.
- Darien I. International Classification of Sleep Disorders – Third Edition: Text Revision (ICSD-3-TR). Darien, IL: American Academy of Sleep Medicine; 2023.
- Brinjikji W, Luetmer PH, Comstock B, et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR Am J Neuroradiol. 2015;36(4):811–816.
- de Zambotti M, Cellini N, Goldstone A, Colrain IM, Baker FC. Wearable sleep technology in clinical and research settings. Med Sci Sports Exerc. 2019;51(7):1538–1557.
- Krystal AD, Edinger JD. Measuring sleep quality. Sleep Med. 2008;9(Suppl 1):S10–S17.
- Esteva A, Robicquet A, Ramsundar B, et al. A guide to deep learning in healthcare. Nat Med. 2019;25(1):24–29.
- Song JK, Christie SD. Minimally invasive cervical stenosis decompression. Neurosurg Clin N Am. 2006;17:423–428.
- Yildiz T, Aydin G, Aksoy S, Karahan AY. Effectiveness of myofascial release on pain, sleep, and quality of life in fibromyalgia syndrome: a systematic review. J Back Musculoskelet Rehabil. 2021;34(2):317–324.
- Zhao D, Ma Y, Wang L, et al. Efficacy and safety of stellate ganglion block with different volumes of ropivacaine for insomnia patients: a randomized controlled trial. BMC Anesthesiol. 2023;23(1):98.
- van Buyten JP, Linderoth B. Does spinal cord stimulation improve sleep disturbances in patients with chronic pain? A literature review. Neuromodulation. 2020;23(6):797–805.
- Esteva A, Robicquet A, Ramsundar B, et al. A guide to deep learning in healthcare. Nat Med. 2019;25:24–29.
- Pigeon WR, Bishop TM, Krueger KM. Insomnia as a precipitating factor in new onset mental illness: a systematic review of recent findings. Curr Psychiatry Rep. 2017;19(8):44.
- Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1(1):40–43.
- Zheng N, Yuan XY, Chi YY, et al. The universal existence of myodural bridge in mammals: an indication of a necessary function. Sci Rep. 2017;7(1):8248.
- Bisson EF, Mummaneni PV, Michalopoulos GD, et al. Sleep disturbances in cervical spondylotic myelopathy: prevalence and postoperative outcomes. Clin Spine Surg. 2023;36(3):112–119.
- Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14(12):1539–1552.
- Rahimizadeh A, Malekmohammadi Z, Karimi M, et al. Unstable os odontoideum contributing to cervical myelopathy and obstructive sleep apnea. Surg Neurol Int. 2019;10:125.
- Alperin N, Lee SH, Loth F, Raksin PB, Lichtor T. Low resistance pathway for cerebrospinal fluid outflow in normal subjects and patients with idiopathic intracranial hypertension. Magn Reson Med. 2000;43(5):735–740.
- Chen Z, Ding Y, Ji X, et al. Long-term safety and efficacy of stenting on correcting internal jugular vein and cerebral venous sinus stenosis. Ann Clin Transl Neurol. 2023;10(8):1305–1313.
- Hack GD, Koritzer RT, Robinson WL, Hallgren RC, Greenman PE. Anatomic relation between the rectus capitis posterior minor muscle and the dura mater. Spine (Phila Pa 1976). 1995;20(23):2484–2486.
- de Wijer A, Steenks MH, de Leeuw JR, et al. Symptoms of cervical spine dysfunction in temporomandibular and cervical spine disorders. J Oral Rehabil. 1996;23(9):742–753.
- Caverzasio S, Amato N, Chiaro G, et al. Impairment of sleep homeostasis in cervical dystonia patients. Sci Rep. 2022;12(1):6866.
- Kyrosis I, Paraskevopoulos E, Koumantakis GA, et al. Heart rate variability in chronic nonspecific neck pain: a cross-sectional study. Healthcare (Basel). 2024;12(11):1055.
- Luthi A. Sleep: Switching off the off-switch. Curr Biol. 2016;26(16):R765–R767.
- Wang ZJ, Li XY, Wang XY, et al. Glucocorticoid receptors in the locus coeruleus mediate sleep disorders caused by repeated corticosterone treatment. Sci Rep. 2015;5:9442.
- Burgess HJ, Trinder J, Kim Y, Luke D. Sleep onset is regulated by melatonin and its suppression is mediated by sympathetic nervous activity. J Clin Endocrinol Metab. 1997;82(10):3643–3648.
- Jarrin DC, Ivers H, Lamy M, Chen IY, Harvey AG, Morin CM. Cardiovascular autonomic dysfunction in insomnia patients with objective short sleep duration. J Sleep Res. 2018;27(3):e12663.
- Bonnet MH, Arand DL. Hyperarousal and insomnia: State of the science. Sleep Med Rev. 2010;14(1):9–15.
- Yamamoto S, Maeda T, Matsumoto M, et al. Sympathetic nerve activation induced by cervical stimulation: its role in sleep regulation. Auton Neurosci. 2002;97(2):123–128.
- Sun SY, Chen GH. Treatment of circadian rhythm sleep–wake disorders. Curr Neuropharmacol. 2022;20(6):1022–1034.
- Khurshid A. A review of changes in DSM-5 sleep–wake disorders. Psychiatr Times. 2015;32(9).
- Sack RL, Auckley D, Auger RR, et al. Circadian rhythm sleep disorders: part I, basic principles, shift work and jet lag disorders. Sleep. 2007;30(11):1460–1483.
- Al Khalili Y, Ly N, Murphy PB. Cervicogenic headache. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Oct 3.
- Matsudaira K, Oka H, Kawaguchi H, et al. Sleep disturbance is associated with neck pain: a 3-year longitudinal study after the Great East Japan Earthquake. BMC Musculoskelet Disord. 2022;23(1):1–9.
- Santos M, Gabani FL, de Andrade SM, Bizzozero-Peroni B, Martinez-Vizcaino V, Gonzalez AD, Mesas AE. The bidirectional association between chronic musculoskeletal pain and sleep-related problems: a systematic review and meta-analysis. Rheumatology (Oxford). 2023;62:2951–2962.
- Pan LM, Hong ZB, Guan RQ. Research progress on insomnia treated by traditional Chinese medicine and acupuncture based on microbial-gut-brain axis theory. World J Clin Cases. 2024;12(18):3314–3320.
- Caverzasio S, Amato N, Chiaro G, Staedler C, Kaelin-Lang A, Galati S. Impairment of sleep homeostasis in cervical dystonia patients. Sci Rep. 2022;12(1):6866.
- Antelmi E, Ferri R, Provini F, et al. Modulation of the muscle activity during sleep in cervical dystonia. Sleep. 2017;40(7):zsx078.
- Hentschel F, Dressler D, Abele M, Paus S. Impaired heart rate variability in cervical dystonia is associated to depression. J Neural Transm (Vienna). 2017;124(2):245–251.
- Samushiya MA, Ragimova AA, Ivolgin AF, et al. Sleep disorders in patients with cervical dystonia. Zh Nevrol Psikhiatr Im S S Korsakova. 2020;120(12):25–29.
- Ray S, Pal PK, Yadav R. Non-motor symptoms in cervical dystonia: a review. Ann Indian Acad Neurol. 2020;23(4):449–457.
- Hack GD, Koritzer RT, Robinson WL, Hallgren RC, Greenman PE. Anatomic relation between the rectus capitis posterior minor muscle and the dura mater. Spine (Phila Pa 1976). 1995;20(23):2484–2486.
- Li C, Yue C, Liu ZC, et al. The relationship between myodural bridges, hyperplasia of the suboccipital musculature, and intracranial pressure. PLoS One. 2022;17:e0273193.
- Xu Q, Shao CX, Zhang Y, et al. Head-nodding: a driving force for the circulation of cerebrospinal fluid. Sci Rep. 2021;11:14233.
- Nedergaard M, Goldman SA. Glymphatic failure as a final common pathway to dementia. Science. 2020;370(6512):50–56.
- Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111.
- Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377.
- Plog BA, Nedergaard M. The glymphatic system in central nervous system health and disease: past, present, and future. Annu Rev Pathol. 2018;13:379–394.
- Yang H, Wei XS, Gong J, et al. The relationship between myodural bridge, atrophy and hyperplasia of the suboccipital musculature, and cerebrospinal fluid dynamics. Sci Rep. 2023;13:18882.
- Alix ME, Bates DK. Fascial manipulation and its role in the treatment of musculoskeletal pain: a review of the literature. J Am Osteopath Assoc. 2011;111(12):687–693.
- Fernández-de-Las-Peñas C, Ambite-Quesada S, Valera-Calero JA, et al. Heart rate variability in women with myofascial pain syndrome: a case-control study. Pain Med. 2021;22(7):1505–1513.
- Haller H, Lauche R, Sundberg T, Dobos G, Cramer H. Craniosacral therapy for chronic pain: a systematic review and meta-analysis of randomized controlled trials. BMC Musculoskelet Disord. 2019;21:1.
- Ughreja RA, Venkatesan P, Gopalakrishna DB, Singh YP, Lakshmi VR. Feasibility and efficacy of craniosacral therapy on sleep quality in fibromyalgia syndrome: a pre-post pilot trial. Int J Ther Massage Bodywork. 2023;16(2):4–11.
- Muñoz-Muñoz S, Muñoz-García MT, Alburquerque-Sendín F, Arroyo-Morales M, Fernández-de-las-Peñas C. Myofascial trigger points, pain, disability, and sleep quality in individuals with mechanical neck pain. J Manipulative Physiol Ther. 2012;35:608–613.
- Liu P, Owashi K, Monnier H, et al. Validating the accuracy of real-time phase-contrast MRI and quantifying the effects of free breathing on cerebrospinal fluid dynamics. Fluids Barriers CNS. 2024;21(1):25.
- Stecco C, Macchi V, Porzionato A, et al. The fascial connections of the cervical region: a basis for manual therapy. Clin Anat. 2011;24(7):723–729.
- de Wijer A, Steenks MH, de Leeuw JR, et al. Symptoms of cervical spine dysfunction in temporomandibular and cervical spine disorders. J Oral Rehabil. 1996;23(11):742–750.
- Manfredini D, Lobbezoo F. Role of psychosocial factors in the etiology of bruxism. J Orofac Pain. 2009;23(2):153–166.
- Armijo-Olivo S, Pitance L, Singh V, et al. The effectiveness of manual therapy and exercise for treating temporomandibular disorders: a systematic review and meta-analysis. J Oral Facial Pain Headache. 2016;30(3):169–184.
- Svensson P, Graven-Nielsen T. The neurobiology of craniofacial muscle pain: review of current knowledge. J Oral Rehabil. 2008;35(7):524–547.
- Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 2013;14(7):502–511.
- Kato T, Thie NM, Huynh N, Miyawaki S, Lavigne GJ. Sleep bruxism and the role of peripheral sensory influences. J Oral Facial Pain Headache. 2003;17(3):191–213.
- Lavigne GJ, Kato T, Kolta A, Sessle BJ. Neurobiological mechanisms involved in sleep bruxism. Crit Rev Oral Biol Med. 2003;14(1):30–46.
- Sanders AE, et al. Subjective sleep quality deteriorates before development of painful temporomandibular disorder. J Pain. 2016;17(6):669–677.
- Slade GD, Bair E, Greenspan JD, et al. Signs and symptoms of first-onset TMD and sociodemographic predictors of its development: the OPPERA prospective cohort study. J Pain. 2013;14(12 Suppl):T20–T32.
- Palomeque-del-Cerro L, Arráez-Aybar LA, Rodríguez-Blanco C, et al. A systematic review of the soft-tissue connections between neck muscles and dura mater: the myodural bridge. Spine (Phila Pa 1976). 2017;42(1):49–54.
- Xiao F, Liu C, Yu S, et al. Magnetic resonance imaging-based classification of the myodural bridge complex with relevant muscles: current status and future perspectives. J Musculoskelet Neuronal Interact. 2020;20(3):386–393.
- Kawaguchi Y, Iida M, Seki S, et al. Os odontoideum with cervical myelopathy due to posterior subluxation of C1 presenting sleep apnea. J Orthop Sci. 2011;16(3):329–333.
- Shirasaki N, Okada K, Oka S, et al. Os odontoideum with posterior atlantoaxial instability. Spine (Phila Pa 1976). 1991;16:706–715.
- Rahimizadeh A, et al. Unstable os odontoideum contributing to cervical myelopathy and obstructive sleep apnea. Surg Neurol Int. 2019;10:125.
- Hall CW, Danoff D. Sleep attacks—apparent relationship to atlantoaxial dislocation. Arch Neurol. 1975;32(1):57–58.
- Zamora-Niño CF, Villafuerte-Trisolini BJ, Vizcarra-Escobar DR. A case of positional central sleep apnea due to compression of the left vertebral artery on brainstem. Sleep Sci. 2018;11(4):211–214.
- Fielding JW, Hawkins RJ. Atlanto-axial rotatory fixation. J Bone Joint Surg Am. 1977;59(1):37–44.
- Fargen KM, Liu K, Garner RM, et al. Recommendations for the selection and treatment of patients with idiopathic intracranial hypertension for venous sinus stenting. J Neurointerv Surg. 2018;10(12):1203–1208.
- Khandelwal N, Kalra N, Garg A, et al. Idiopathic intracranial hypertension: imaging and clinical fundamentals. Neuroradiol J. 2020;33(1):66–70.
- Fultz NE, Bonmassar G, Setsompop K, et al. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science. 2019;366(6465):628–631.
- Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377.
- Alperin N, Lee SH, Sivaramakrishnan A, et al. Quantifying the effect of posture on intracranial physiology in humans by MRI flow studies. J Magn Reson Imaging. 2005;22(5):591–596.
- Fargen KM. Venous sinus stenting for idiopathic intracranial hypertension: lessons learned from a high-volume practice. J Neurointerv Surg. 2022;14(6):528–532.
- Fulton AT, Tagg NT, Gospe SM, et al. Correlation between lumbar puncture opening pressure and venous sinus pressure gradient in idiopathic intracranial hypertension (IIH). Interv Neuroradiol. 2025 Apr 24. d
- Satti SR, Vance AZ, Fowler DR, Ulrich CT, Thomas AJ, Lee CC. Venous sinus stenting for idiopathic intracranial hypertension: a review of the literature. J Neurointerv Surg. 2020;12(4):363–368.
- Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111.
- Plog BA, Nedergaard M. The glymphatic system in central nervous system health and disease: past, present, and future. Annu Rev Pathol. 2018;13:379–394.
- Ringstad G, Eide PK. Cerebrospinal fluid tracer efflux to parasagittal dura in humans. Nat Commun. 2020;11(1):354.
- Yin J, Huang X, Shrestha S, et al. Venous outflow impairment and glymphatic dysfunction: a multimodal MRI study in idiopathic intracranial hypertension. Neurology. 2023;101(15):e1480–e1491.
- Hablitz LM, Vinitsky HS, Sun Q, Staeger FF, Sigurdsson B, Mortensen KN, Lilius TO, Nedergaard M. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci Adv. 2019;5:eaav5447.
- Strand FL, Wang L, Paske WJ, et al. The role of the dura mater in cranial pain and headache. Med Hypotheses. 2007;68(3):454–457.
- Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;141(2):122–131.
- Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;141:122–131.
- Chouchou F, Desseilles M. Heart rate variability: a tool to explore the sleeping brain? Front Neurosci. 2014;8:402.
- Milenovic N, Klasnja A, Skrbic R, et al. Sleep problems and disabilities of the arm, shoulder, and hand in persons with thoracic outlet syndrome: a cross-sectional study. Int J Environ Res Public Health. 2022;19(19):12486.
- Cleveland Clinic. Venous thoracic outlet syndrome: causes and symptoms. Cleveland Clinic. 2022.
- Sagirov AF, Sergeev TV, Shabrov AV, et al. Postural influence on intracranial fluid dynamics: an overview. J Physiol Anthropol. 2023;42(1):5.
- Milenovic N, et al. Sleep problems and disabilities of the arm, shoulder, and hand in patients with thoracic outlet syndrome. Int J Environ Res Public Health. 2022;19(19):12486.
- Sic A, Bogicevic M, Brezic N, Nemr C, Knezevic NN. Chronic stress and headaches: the role of the HPA axis and autonomic nervous system. Biomedicines. 2025;13(2):463.
- Shahidi B, Sannes T, Laudenslager M, Maluf KS. Cardiovascular responses to an acute psychological stressor are associated with the cortisol awakening response in individuals with chronic neck pain. Physiol Behav. 2015;150:93–98.
- Leistad RB, Stovner LJ, White LR, Nilsen KB, Westgaard RH, Sand T. Noradrenaline and cortisol changes in response to low-grade cognitive stress differ in migraine and tension-type headache. J Headache Pain. 2007;8:157–166.
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89.
- Vgontzas AN, Bixler EO, Lin HM, et al. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2001;86(8):3787–3794.
- Grunstein RR, Sullivan CE. Sleep apnea and hypothyroidism: mechanisms and management. Am J Med. 1988;85(5):775–779.
- Kraemer S, Danker-Hopfe H, Dorn H, Anderer P, Ehrenthal JC. Thyroid function and sleep: a complex relationship. J Thyroid Res. 2011;2011:420580.
- Baker FC, Lampio L, Saaresranta T, Polo-Kantola P. Sleep and sleep disorders in the menopausal transition. Sleep Med Clin. 2018;13(3):443–456.
- Shahidi B, Sannes T, Laudenslager M, Maluf KS. Cardiovascular responses to an acute psychological stressor are associated with the cortisol awakening response in individuals with chronic neck pain. Physiol Behav. 2015;150:93–98.
- Mbiydzenyuy NE, Qulu LA. Stress, hypothalamic-pituitary-adrenal axis, hypothalamic-pituitary-gonadal axis, and aggression. Metab Brain Dis. 2024;39:1613–1636.
- Williams DP, Koenig J, Carnevali L, et al. Heart rate variability and inflammation: a meta-analysis of human studies. Brain Behav Immun. 2019;80:219–226.
- Bernardini R, Kamilaris TC, Calogero AE, et al. Interactions between tumor necrosis factor-alpha, hypothalamic corticotropin-releasing hormone, and adrenocorticotropin secretion in the rat. Endocrinology. 1990;126(6):2876–2881.
- Kim JH, Kim JH, Kim TH. Changes in sleep problems in patients who underwent surgical treatment for degenerative spinal disease with a concurrent sleep disorder: a nationwide cohort study in 3183 patients during a two-year perioperative period. J Clin Neurosci. 2022;100:1–10.
- Esteva A, Robicquet A, Ramsundar B, et al. A guide to deep learning in healthcare. Nat Med. 2019;25(1):24–29.
- Moeini M, Gorji HA, Dehghani A. Evaluation of cerebral blood flow and brain activity changes in patients with insomnia using multimodal imaging: a systematic review. J Clin Neurosci. 2022;100:1–10.
- Shickel B, Tighe PJ, Bihorac A, Rashidi P. Deep EHR: a survey of recent advances in deep learning techniques for electronic health record (EHR) analysis. IEEE J Biomed Health Inform. 2018;22(5):1589–1604.
- Chun-Yiu JP, Man-Ha ST, Chak-Lun AF. The effects of pillow designs on neck pain, waking symptoms, neck disability, sleep quality and spinal alignment in adults: a systematic review and meta-analysis. Clin Biomech (Bristol). 2021;85:105353.
- Satti SR, Vance AZ, Fowler DR, et al. Venous sinus stenting for idiopathic intracranial hypertension: a review of the literature. J Neurointerv Surg. 2020;12(4):363–368.
- Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1(1):40–43.