Translational Perioperative and Pain Medicine (ISSN: 2330-4871)
ARTICLE DOI: 10.31480/2330-4871/181
Research Article | Volume 10 | Issue 3 Open Access
Perioperative Hyperglycemia: An Independent Risk Factor for Adverse Outcomes in Patients Undergoing Ascending Aorta Repair
Stephanie Bradley, MD, MPH1, Travis Markham, MD1, Shaodi Yan, MD, PhD1, Medea Mshvildadze, MD1, Harleen K. Sandhu, MD, MPH2, Charles C. Miller 3rd, PhD2, Anthony L. Estrera, MD2 and Shao Feng Zhou, MD1*
1Department of Cardiothoracic and Vascular Anesthesiology, McGovern Medical School at UTHealth Houston, Houston, Texas, USA
2Department of Cardiothoracic and Vascular Surgery, McGovern Medical School at UTHealth Houston, Houston, Texas, USA
Shao Feng Zhou, MD, Department of Anesthesiology, McGovern Medical School at UTHealth Houston, 6431 Fannin St., MSB. 5.020 Houston, TX, USA, Tel: +1-713-500-6201, E-mail: shao.feng.zhou@uth.tmc.eduEditor: Renyu Liu, MD; PhD; Professor, Department of Anesthesiology and Critical Care, Perelman School of Medicine at the University of Pennsylvania, Center of Penn Global Health Scholar, 336 John Morgan building, 3620 Hamilton Walk, Philadelphia, PA 19104, USA, Fax: 2153495078, E-mail: RenYu.Liu@pennmedicine.upenn.edu
Received: September 08, 2023 | Accepted: November 14, 2023 | Published: November 27, 2023
Citation: Bradley S, Markham T, Yan S, Mshvildadze M, Sandhu HK, et al. Perioperative Hyperglycemia: An Independent Risk Factor for Adverse Outcomes in Patients Undergoing Ascending Aorta Repair. Transl Perioper Pain Med 2023; 10(3):552-559
Abstract
Objective: Hyperglycemia is common in patients undergoing cardiac surgery and has been shown to be associated with worse outcomes. This study investigates if perioperative hyperglycemia has an impact on clinical outcomes on ascending aorta/arch repair surgery.
Methods: We retrospectively reviewed 1,582 cases of ascending aorta/arch repair at a single institution from 2003-2019. Patients were stratified according to level of preoperative and postoperative blood glucose. Chi-Square test and t-test were used for comparison of categorical variables and normally distributed variables between the groups. Multiple groups were compared by the Kruskal-Wallis test. Multivariate logistic regression analysis was used for analysis of the correlation between perioperative glucose and patient outcomes.
Results: In all, 7.3% of patients died in-hospital, 71.0% were discharged to home, and 21.6% discharged to long-term care/rehabilitation facilities. With elevating glucose level before and/or after surgery, in-hospital mortality increased. Higher preoperative (≧ 140 mg/dl) and/or postoperative (≧ 200 mg/dl) glucose measurements are independent predictors for in-hospital mortality (OR/1.98, 95% CI/1.14-3.42, p = 0.015 & OR/2.53, 95% CI/1.37-4.70, p = 0.003) after adjusting for demographic factors, diabetes mellitus, creatinine clearance and surgical variables. Additionally, high preoperative glucose levels are related to lower incidence of discharging home compared to lower preoperative glucose (OR = 0.68, 95% CI = 0.50-0.92, p = 0.013 and OR = 0.60, 95% CI = 0.42-0.87, p = 0.007).
Conclusion: High perioperative glucose is an independent risk factor for in-hospital mortality in patients undergoing ascending aorta repair. Further studies are necessary to confirm this finding and determine the causality.
Keywords
Ascending aorta and aortic arch repair, Deep hypothermic circulatory arrest, Hyperglycemia, Perioperative glucose, In-hospital mortality
Glossary of Terms
AKI: Acute Kidney Injury; ANOVA: Analysis of Variance; aORs: adjusted Odds Ratio; BMI: Body Mass Index; CABG: Coronary Artery Bypass Graft; CBP: Cardiopulmonary Bypass; CrCl: Creatinine Clearance; DHCA: Deep Hypothermic Circulatory Arrest; DM: Diabetes Mellitus; ICU: Intensive Care Unit; LOS: Length of Stay
Introduction
Hyperglycemia is reported in up to 60-90% of patients undergoing cardiac surgery [1]. This hyperglycemia is likely a metabolic consequence of surgical stress and anesthesia [1-4]. Previous studies have shown that perioperative hyperglycemia is related to poor outcomes in cardiac surgical patients [2-5]. All large studies to date have involved patients who underwent congenital heart surgery, non-selective surgery, or coronary artery bypass graft (CABG) [1]. There is a paucity of studies specifically focused on the ascending aorta and aortic arch repair. Patients undergoing ascending aorta and aortic arch repairs present the unique challenge of requiring cardiopulmonary bypass (CPB), often with the addition of deep hypothermic circulatory arrest (DHCA). Due to many of the factors of the surgery, including CPB, DHCA, and surgical stress, hyperglycemia is often noted perioperatively. Therefore, it is prudent to examine the impact of patients’ blood glucose on clinical outcome in this patient population [1,5]. The objective of this study was to evaluate the predictive value of perioperative glucose on the in-hospital outcomes of patients who underwent ascending aorta and aortic arch repair surgery.
Materials and Methods
Patient population and data collection
All patients undergoing cardiac surgery at Memorial Hermann Heart & Vascular Institute between January 2003 and December 2019 were retrospectively reviewed. The database was established by collecting medical information through patient medical records and International Classification of Diseases-Ninth Revision-Clinical Modification (ICD-9-CM and ICD-10-CM) Volume 1 Diagnosis Codes, including demographic parameters, medical history, laboratory test results, medical treatment, procedural information, complications, and clinical outcomes.
From 2003-2019, a total of 1,760 patients underwent ascending aorta or aortic arch repair in our institution with one surgical team. In all, 1,582 patients were included in this study, while the remaining 178 were excluded due to incomplete data. Patients were analyzed according to their preoperative (< 110 mg/dl, 110-139 mg/dl, and ≥ 140 mg/dl) and postoperative (< 140 mg/dl, 140-199 mg/dl, and ≥ 200 mg/dl) blood glucose levels and placed into the appropriate category. The McGovern Medical School at UTHealth Houston Committee for the Protection of Human Subjects approved this retrospective study and consent was waived (IRB HSC-MS-18-0371, June 6, 2018). After data was queried and extracted through an electronic database, it was manually verified for accuracy by study assistants, and any missing information was obtained from alternate sources in the electronic medical record for all cases that were collected. This process provided an accurate and validated dataset for analysis.
Surgical procedures and glucose control
At our institution, hyperglycemia control is standardized with mortified Atlanta Insulin Infusion Protocol intraoperatively among the cardiovascular anesthesiologist performing the anesthetic for the surgery. The consensus is to treat all intraoperative glucose values above 180 mg/dL with either a bolus of insulin and/or continuous insulin infusion. We targeted a blood glucose below 200 mg/dL and above 150 md/dL based on frequent arterial blood gases. This was an institutional standardized management followed by each anesthesiologist. In the postoperative phase, blood glucose management follows the Atlanta Insulin Infusion Protocol in the intensive care unit (ICU), where an insulin drip is started and titrated accordingly, based on hourly glucose draws with oversight provided by a critical care physician.
Definitions
Preoperative glucose was defined in this study as fasting glucose in elective patients or the most proximal random glucose in emergency/urgent surgical patients prior to entering the operating room. Postoperative glucose was defined as glucose tested immediately after arriving to ICU. In-hospital mortality was defined as death from all causes during intraoperative and postoperative hospitalization. Long-term care was defined as a transfer to long-term care/rehabilitation facility, skilled nursing facility, or hospice facility. In order to reduce the impact of multiple factors, causes, and diagnostic criteria of acute kidney injury (AKI), we choose a very extreme point of AKI that needed postoperative HD for oliguria, as defined as “AKI” in this study. Arrhythmia was defined as any new-onset cardiac arrhythmias documented after surgery. Respiratory insufficiency was defined as prolonged ventilator support > 24 hours, the development of acute respiratory distress syndrome, pulmonary edema, pneumonia, or re-intubation. Encephalopathy was confirmed by a neurologist in patients with abnormal level of consciousness manifested as confusion, delirium, agitation, seizures, or faulty cognition.
Statistical analysis
Categorical variables were reported as frequency (percentage), and continuous variables were expressed as mean ± SD, or as median (interquartile range). Chi-Square test was used to compare categorical variables. To assess normally distributed and non-normally distributed continuous variables between two groups, independent samples t-test and Kruskal-Wallis test were applied, respectively. Multiple groups were compared using one-way analysis of variance (ANOVA) or the Kruskal-Wallis test, as appropriate. Multivariate logistic regression analysis and adjusted odds ratios (aORs) were used to evaluate the predictive ability of perioperative glucose level on mortality and discharge disposition. A p-value < 0.05 was considered statistically significant. All analyses were performed with SPSS software version 22.0 (IBM Corp., Armonk, NY) and SAS version 9.2 (SAS Institute Inc, Cary, NC).
Results
A total of 1,582 patients were included in this 17-year study. The clinical characteristics of all enrolled patients are listed in Table 1. The mean age was 57.6 ± 14.9 years, 66.9% were males, the mean creatinine clearance rate (CrCl) was 93.7 ± 44.4 ml/min, and 12.3% had diabetes mellitus (DM). Of all patients, 43.4% had Stanford type A dissection, 31.0% required emergency/urgent operation, 87.5% were performed with DHCA, and the mean operation time was 5.4 ± 2.8 hours. The median (interquartile) ICU length of stay (LOS) was 7.2 days (4.0, 13.2) and median hospital LOS 12.0 (8.3, 19.0) days. In-hospital mortality occurred in 7.3% of patients, and 71.0% of patients were discharged home from the hospital.
Table 1: Characteristics of overall patients and patients stratified by preoperative and postoperative glucose respectively.
| Total Cases | Preoperative Glucose Level (mg/dl) | Postoperative Glucose Level (mg/dl) | |||||||||||
| < 110 | 110-139 | ≥ 140 mg/dl | p-value | < 140 | 140-199 | ≥ 200 mg/dl | p-value | ||||||
| Cases number (% total population) | 1,582 | 978 (61.82%) | 366 (23.14%) | 238 (15.04%) | 420 (26.55%) | 947 (59.86%) | 215 (13.59%) | ||||||
| Age (years) | 57.6 ± 14.9 | 56.4 ± 15.5 | 58.9 ± 13.9 | 60.1 ± 13.2 | < 0.001 | 52.7 ± 16.0 | 59.0 ± 14.3 | 60.7 ± 13.0 | < 0.001 | ||||
| Male | 1,060 (66.9%) | 652 (66.6%) | 251 (68.4%) | 157 (66.0%) | 0.749 | 265 (62.9%) | 646 (68.1%) | 149 (69.3%) | 0.132 | ||||
| BMIa | 28.8 ± 6.5 | 28.1 ± 6.2 | 29.7 ± 6.5 | 30.5 ± 6.8 | < 0.001 | 27.8 ± 5.8 | 29.1 ± 6.7 | 29.7 ± 6.1 | 0.001 | ||||
| CrClb (ml/min) | 93.7 ± 44.4 | 97.6 ± 45.9 | 91.0 ± 41.8 | 81.5 ± 39.5 | < 0.001 | 97.6 ± 47.9 | 92.1 ± 43.0 | 92.9 ± 43.4 | 0.106 | ||||
| Baseline Medical Conditions | |||||||||||||
| DM | 194 (12.3%) | 78 (8.0%) | 46 (12.6%) | 70 (29.4%) | < 0.001 | 27 (6.4%) | 118 (12.5%) | 49 (22.8%) | < 0.001 | ||||
| HTN | 1,129 (71.4%) | 687 (70.2%) | 261 (71.3%) | 181 (76.1%) | 0.206 | 291 (69.3%) | 686 (72.4%) | 152 (70.7%) | 0.479 | ||||
| CAD | 242 (15.3%) | 144 (14.7%) | 59 (16.1%) | 39 (16.4%) | 0.720 | 44 (10.5%) | 150 (15.8%) | 48 (22.3%) | < 0.001 | ||||
| COPD | 263 (16.6%) | 160 (16.4%) | 72 (19.7%) | 31 (13.0%) | 0.094 | 67 (16.0%) | 164 (17.3%) | 32 (14.9%) | 0.626 | ||||
| ESRD | 80 (5.1%) | 39 (4.0%) | 21 (5.7%) | 20 (8.4%) | 0.016 | 18 (4.3%) | 48 (5.1%) | 14 (6.5%) | 0.480 | ||||
| Surgical Variables | |||||||||||||
| Type A dissection | 687 (43.4%) | 304 (31.1%) | 214 (58.5%) | 169 (71.0%) | < 0.001 | 177 (42.1%) | 399 (42.1%) | 111 (51.6%) | 0.033 | ||||
| Emergent/Urgent | 491 (31.0%) | 173 (17.7%) | 172 (47.0%) | 146 (61.3%) | < 0.001 | 129 (30.5%) | 290 (30.6%) | 73 (34.0%) | 0.609 | ||||
| DHCA | 1,385 (87.5%) | 845 (86.4%) | 328 (89.6%) | 212 (89.1%) | 0.209 | 358 (85.2%) | 844 (89.1%) | 183 (85.1%) | 0.068 | ||||
| Operation time (hour) | 5.4 ± 2.8 | 5.2 ± 2.4 | 5.7 ± 3.4 | 5.9 ± 3.1 | < 0.001 | 5.4 ± 2.4 | 5.4 ± 2.9 | 5.7 ± 3.0 | 0.346 | ||||
| Pump time (minute) | 156.5 ± 54.4 | 154.2 ± 56.4 | 160.4 ± 51.8 | 159.8 ± 49.6 | 0.112 | 160.1 ± 51.6 | 154.5 ± 54.6 | 158.5 ± 58.7 | 0.177 | ||||
| Clamp time (minute) | 97.5 ± 40.4 | 96.7 ± 42.2 | 98.1 ± 38.1 | 100.0 ± 36.2 | 0.506 | 101.6 ± 41.2 | 95.7 ± 39.8 | 97.2 ± 41.1 | 0.044 | ||||
| Clinical Outcomes | |||||||||||||
| Ventilation Time (day) | 1582 | 2 (1,3) | 2 (1, 6) | 3 (2,7) | < 0.001 | 2 (1,4) | 2 (1,4.75) | 2 (2, 5) | 0.023 | ||||
| Respiratory insufficiencyc | 445 (30.4%) | 213 (23.0%) | 130 (38.7%) | 102 (50.2%) | < 0.001 | 120 (30.3%) | 261 (29.4%) | 64 (35.2%) | 0.304 | ||||
| Arrhythmiac | 577 (39.4%) | 362 (39.1%) | 124 (36.9%) | 91 (44.8%) | 0.180 | 136 (34.3%) | 362 (40.8%) | 79 (43.4%) | 0.046 | ||||
| Acute kidney injuryc | 290 (19.8%) | 148 (16.0%) | 91 (27.1%) | 51 (25.1%) | < 0.001 | 68 (17.2%) | 172 (19.4%) | 50 (27.5%) | 0.014 | ||||
| Encephalopathyc | 97 (6.6%) | 49 (5.3%) | 32 (9.5%) | 16 (7.9%) | 0.020 | 26 (6.6%) | 56 (6.3%) | 15 (8.2%) | 0.632 | ||||
| Length of stayd | 12.0 (8.3,19.0) | 11.3 (8.0,17.2) | 13.8 (9.0,20.8) | 14.8 (9.1,20.8) | < 0.001 | 11.5 (8.0,19.4) | 12.3 (8.3,18.7) | 12.6 (8.5,19.7) | 0.445 | ||||
| ICU Length of stayd | 7.2 (4.0, 13.2) | 6.9 (3.8, 6.9) | 8.0 (4.3, 8.0) | 9.0 (5.1, 17.0) | < 0.001 | 6.9 (3.5, 11.8) | 7.3 (4.0, 13.8) | 8.2 (4.5, 13.9) | 0.029 | ||||
| Discharge home | 1125 (71.0%) | 762 (77.9%) | 234 (63.9%) | 129 (54.2%) | < 0.001 | 320 (76.2%) | 666 (70.3%) | 139 (64.7%) | 0.007 | ||||
| Discharge to long-term care | 341 (21.6%) | 165 (16.9%) | 102 (27.9%) | 74 (31.1%) | < 0.001 | 76 (18.1%) | 222 (23.4%) | 43 (20.0%) | 0.072 | ||||
| In-hospital death | 116 (7.3%) | 51 (5.2%) | 30 (8.2%) | 35 (14.7%) | < 0.001 | 24 (5.7%) | 59 (6.2%) | 33 (15.3%) | < 0.001 | ||||
Abbreviations: BMI: Body Mass Index; CAD: Coronary Artery Disease; Ccr: Creatinine Clearance; COPD: Chronic Obstructive Pulmonary Disease; DHCA: Deep Hypothermic Circulatory Arrest; DM: Diabetes Mellitus; ESRD: End Stage Renal Disease; OR: Operation
aBMI is the weight in kilograms divided by the square of the height in meters; bCrCl was calculated using the Cockcroft-Gault equation: CrCl = ([140-age] × weight in kg × 0.85 if female/(serum creatinine × 72); cStatistical analysis was performed in discharged patients (n = 1,466); dLOS was expressed as median (interquartile range).
The distribution of preoperative and postoperative glucose levels are shown in Figure 1. Postoperative glucose (161.7 ± 46.4 mg/dl) was higher than the preoperative glucose (111.3 ± 37.3 mg/dl) in this patient population (p < 0.001). The correlation between the two variables was assessed by Pearson correlation. The result showed that there was a small positive correlation between the blood glucose levels before and after the operation (r = 0.116, p < 0.001; Figure 2). The comparison of baseline characteristics among groups with different levels of preoperative glucose (< 110 mg/dl, 110-139 mg/dl, and ≧ 140 mg/dl) or postoperative glucose (< 140 mg/dl, 140-199 mg/dl, and ≧ 200 mg/dl) is shown in Table 1. Patients with higher perioperative glucose were older, had higher body mass index (BMI), lower creatinine clearance (CrCl), higher rates of preexisting DM, longer operation time, and higher frequency of emergency or urgent surgery.
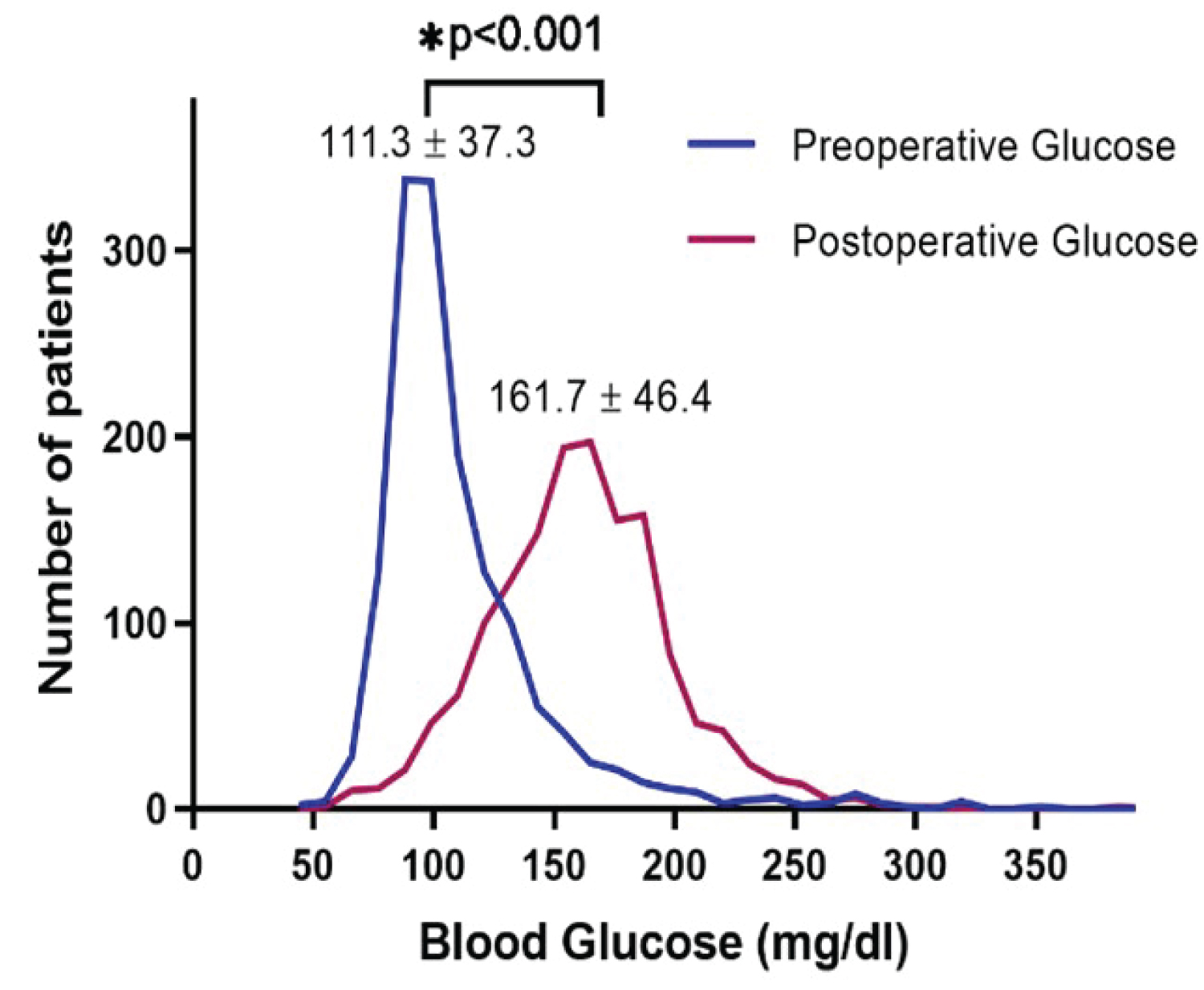
Figure 1: Distribution trend of average blood glucose perioperative.
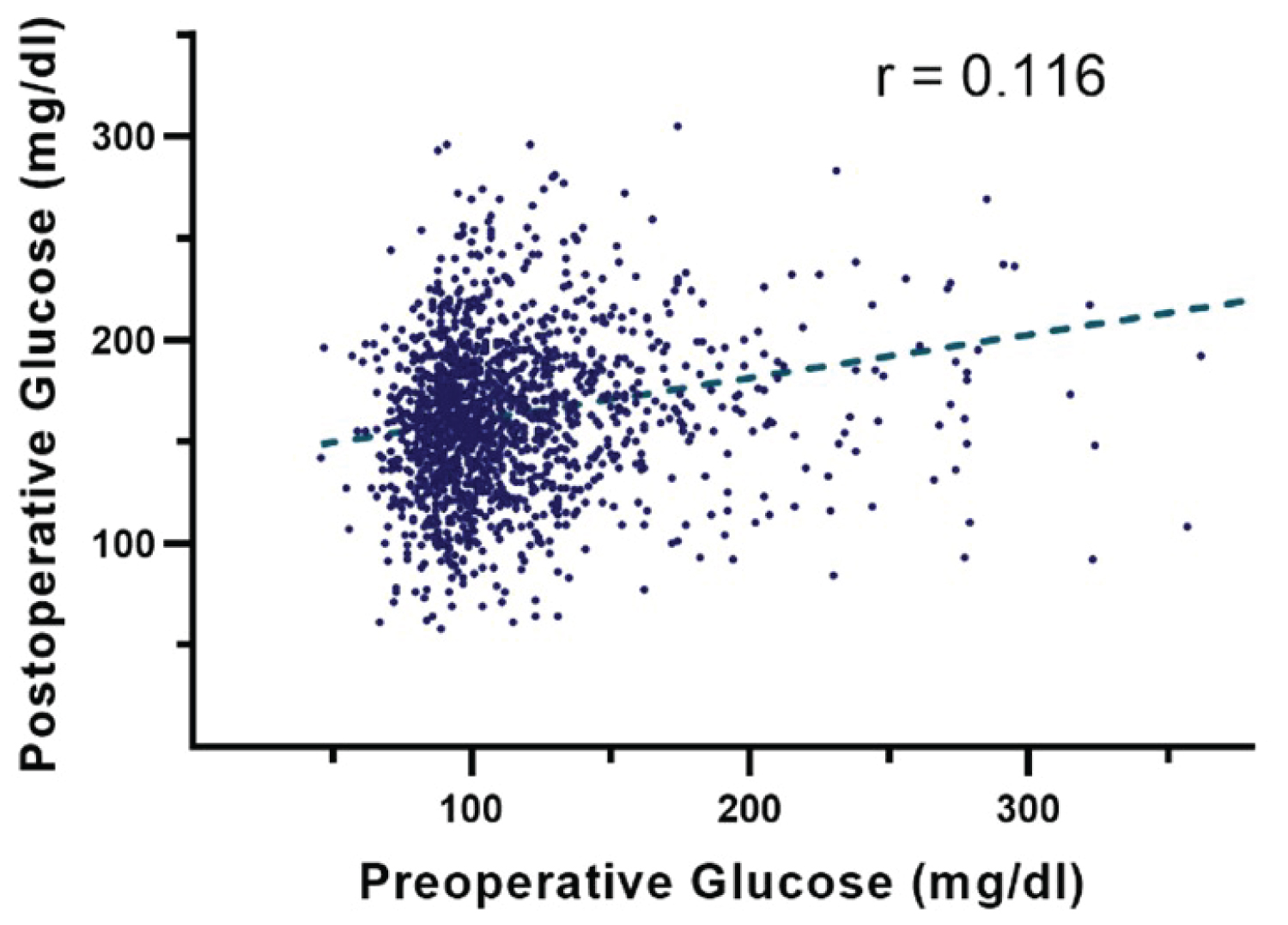
Figure 2: Potential weak correlation between preoperative and postoperative glucose levels (r = 0.116, p < 0.001).
Patients with higher preoperative glucose levels had higher rates of respiratory insufficiency, AKI, and encephalopathy in the postoperative phase. Patients with higher postoperative glucose were also more likely to experience AKI and develop arrhythmias. In looking at survival, patients with higher perioperative glucose levels had a higher risk of in-hospital death and lower rates of discharge to home.
The rates of discharged home and in-hospital death in different groups stratified by the combination of preoperative and postoperative glucose are summarized in Figure 3. Patients with preoperative glucose < 110 mg/dl and postoperative glucose < 140 mg/dl had the highest discharged home rate and lowest in-hospital mortality. As preoperative and postoperative glucose levels increased, in-hospital mortality increased and discharge to home rate decreased.
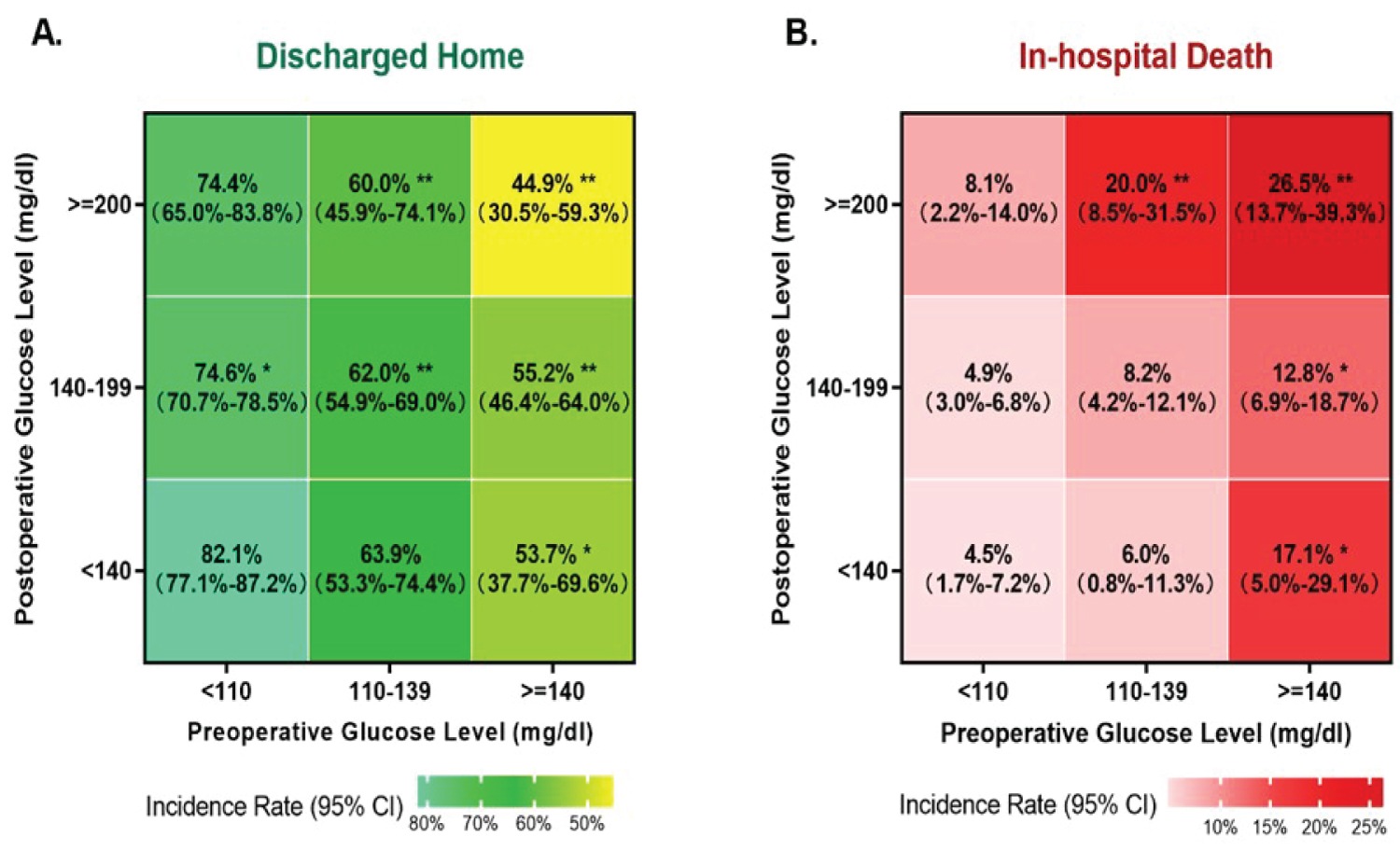
Figure 3: The combined impact on preoperative glucose (X axis) and postoperative glucose (Y axis) on the rates of discharged home (A) and in-hospital death (B). *p-value < 0.05 and **p-value < 0.001 compared to the group with preoperative glucose < 110 mg/dl and postoperative glucose < 140 mg/dl.
High preoperative (≧ 140 mg/dl) and postoperative (≧ 200 mg/dl) glucose are independent predictors for in-hospital mortality (preop OR = 1.98, 95% CI = 1.14-3.42, p = 0.015: postop OR = 2.53, 95% CI 1.37-4.70, p = 0.003) after adjusting for demographic factors (age, gender, BMI), diabetes mellitus history, creatinine clearance and surgical variables (emergency/urgent operation, DHCA, operation time, pump time and clamp time). Additionally, higher preoperative glucose levels (110-139 mg/dl and ≧ 140 mg/dl) are also related to lower incidence of discharging home compared to lower preoperative glucose < 110 mg/dl (preop OR = 0.68, 95% CI = 0.50-0.92, p = 0.013; postop OR = 0.60, 95% CI = 0.42-0.87, p = 0.007) (Figure 4). ICU LOS (9.0 days [5.1, 17.0]) and hospital LOS (14.8 days [9.1, 20.8]) were also statistically significant for patients with elevated preoperative glucose > 140 mg/dL. ICU LOS and Hospital LOS were not statistically significant different for patients with elevated postoperative glucose.
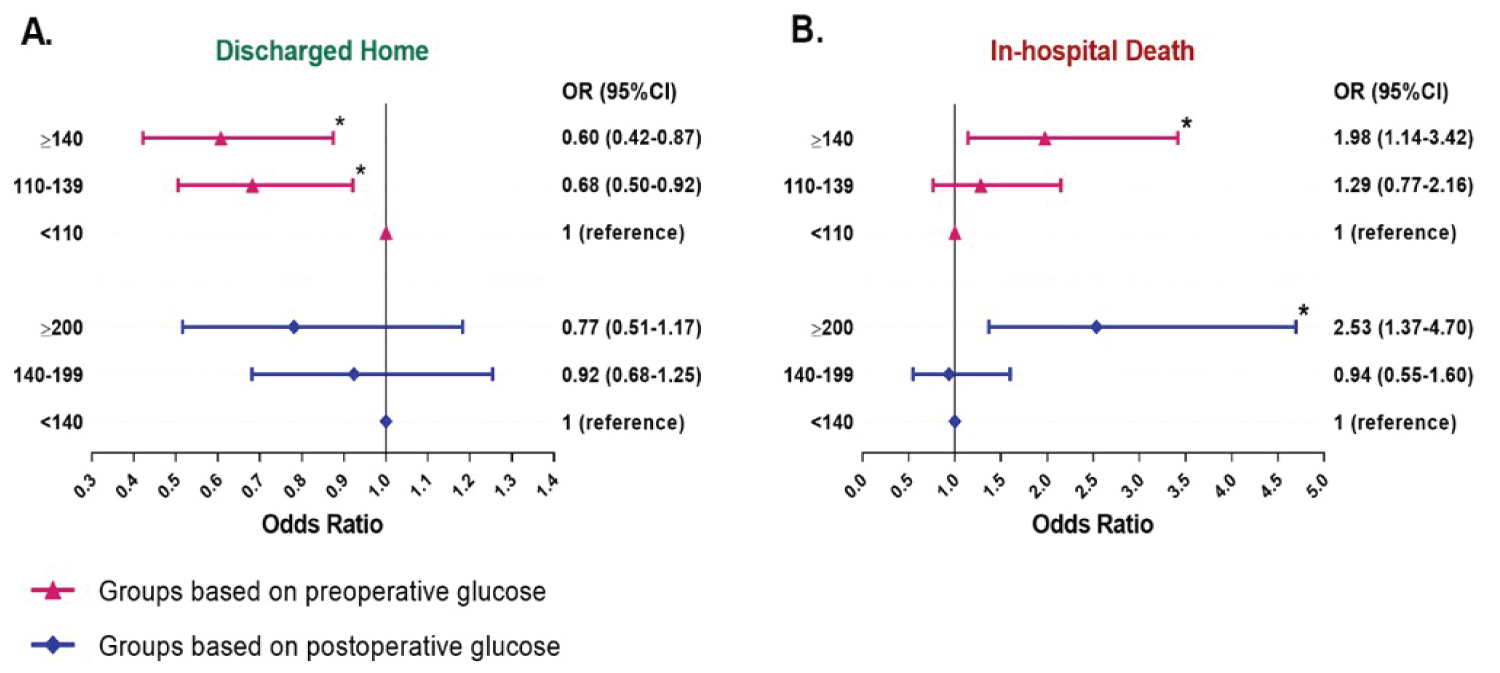
Figure 4: Results of multivariate logistic regression analysis for the in-hospital mortality (A) and discharged home (B) in patients stratified by preoperative and postoperative glucose respectively. Odds ratios (ORs) were adjusted for demographic factors (age, gender, body mass index), diabetes mellitus history, creatinine clearance and surgical variables (emergency/urgent operation, operation time, pump time and clamp time). *p-value < 0.05.
Discussion
Perioperative hyperglycemia was common in patients included in the study, despite standardized glycemic control management. In all, 16.3% of patients had preoperative glucose values higher than 140 mg/dl. Following surgery, 82.9% of patients had glucose values greater than 140 mg/dl, with 15.7% having values greater than 200 mg/dl.
Multiple factors determine the preoperative glucose of a patient, including comorbidities, the level of glucose control prior to hospitalization. , disease severity, and home medications with DM patient [6] interestingly, this data suggests that it is unlikely that blood glucose after surgery is strongly associated with the preoperative glucose level. The study shows only a weak positive correlation between preoperative and postoperative blood glucose (Pearson’s r = 0.171).
Postoperative glucose is primarily affected by surgical stress, operation time, surgery invasiveness, CPB, and DHCA [4,7-9]. Therefore, in addition to the clinical application of current and novel methods that have shown potential effectiveness [6,10], the advocacy of integrated management is critical to future glucose control.
Our data suggests that higher perioperative glucose is independently associated with higher rates of in-hospital mortality and lower rates of discharge to home, regardless of demographic factors, history of DM, creatinine clearance, or other surgical variables. This study is consistent with previous studies conducted in other types of cardiac surgery [2-4,11-14]. Thiele, et al. showed, via retrospective analysis, that admission blood glucose is correlated with increased morbidity and mortality among patients undergoing emergency CABG surgery [12]. In a retrospective analysis, Ascione, et al. showed that inadequate postoperative blood glucose control is a predictor of in-hospital mortality and morbidity [11].
There are several possible mechanisms that may explain this observation. First, hyperglycemia can enhance the glucose toxicity and oxidative stress reaction in the body, which can increase tissue damage and insulin resistance, a theory discussed by Li, et al. [14]. Second, high blood sugar may activate blood coagulation and promote osmotic diuresis, which, together, increase the risk of thromboembolism, ultimately leading to poor outcomes [15,16]. Additionally, hyperglycemia is associated with increased lipolysis and excess circulating free fatty acids, which are toxic to ischemic myocardium and may lead to damaged cell membranes in the myocardium, arrhythmias, calcium overload, increase myocardial oxygen demands and reduced contractility [15,17,18]. Other possible explanations include inflammation activation, the abolishment of intrinsic myocardial protective mechanisms, or increased infection [15,19,20]. From this evidence, it is reasonable to believe that hyperglycemia is not only a biomarker of poor clinical outcomes but also, potentially, a strong contributor to poor clinical outcomes.
The current guidelines from the Society of Thoracic Surgeons recommends that diabetic patients maintain blood glucose concentrations ≤ 180 mg/dL before surgery and patients without diabetes have no recommended management [2]. In this study, we found that preoperative glucose ≥ 110 mg/dl is associated with longer ICU or hospital LOS, worse discharge status, and higher mortality (Table 1). After multivariate adjustment, preoperative glucose ≥ 110 mg/dl and preoperative glucose ≥ 140 mg/dl remained risk factors for in-hospital mortality-and these patients were less likely to be discharged to home (Figure 4). These results suggest that patients undergoing ascending aortic or aortic arch repair may benefit from more strict preoperative glucose control than currently recommended guidelines. Additionally, these results suggest that non-diabetic patients also need standardized blood glucose management prior to surgery.
Postoperative glucose in this study was measured immediately after surgery and, thus, it largely reflects intraoperative glucose management. A randomized trial by Gandhi, et al. evaluated the effects of intraoperative insulin in cardiac surgery patients, and their results showed no significant difference in primary outcomes between conventional group targeted to keep serum glucose < 200 mg/dl and intensive insulin therapy maintain serum glucose between 80 and 100 mg/dL [21]. However, we found that patients with postoperative glucose ≥ 200 mg/dl are more likely to have arrythmia, AKI, LOS in ICU, in-hospital mortality and less likely to be discharged to home.
The strengths of this study include that it is a single center study, therefore, there is less variability of care between surgeons, anesthesiologists, and ICU care based on hospital volume. Additionally, this was a large study, with 1,582 patients, who underwent specifically ascending aorta and aortic arch repair surgery, many with deep hypothermic circulatory arrest.
One limitation is that it is a retrospective review, causing it to be subjected to selection bias. Another limitation is the inclusion of both diabetic and non-diabetic patients, as it is possible that these patients should be looked at in separate cohorts. Further analysis is likely required to determine the optimal glycemic goal in different patient populations and, more specifically, between patients who are diabetic vs. those with no history of DM. Additionally, this study spans 16 years and it is possible the surgical, anesthetic, and critical care techniques have changed over the course of this time, confounding our results.
With limited data from randomized control trials on the benefits of stringent intraoperative glucose control, the authors recommend maintaining blood glucose within a relatively loose range avoiding hypoglycemia [22]. While this study does not prove causality between perioperative hyperglycemia and clinical outcomes, our findings highlight the necessity to further examine the role of hyperglycemia in risk-adjusted outcomes and present an important potential opportunity to improve outcomes for patients undergoing ascending aorta and aortic arch repair surgery.
The ideal range for glycemic control remains unclear, and may differ across populations. However, given the results of this study, as well as prior studies that demonstrate a strong association with hyperglycemia and adverse events, the importance of perioperative glucose control, especially for patients with an extremely high level of glucose, should continue to be emphasized. Additionally, it is possible that guidelines should differentiate between different patient populations regarding blood glucose perioperatively.
Continuous blood glucose monitoring by using artificial endocrine pancreas intraoperatively is revealed by a spike in glucose levels immediately after DHCA on cardiopulmonary bypass [23]. Hopefully, continuous blood glucose monitoring can be used routinely in the perioperative period in order to help better control glucose levels and reduce complications associated hyperglycemia.
Weakness on this study is we don’t have completed information of Preoperative HbA1c levels so that we are unable to estimated how good glucose are controlled before the operation in knowing and unknowing patients with DM. further studies are needed. However, this study is mainly analysis of stress induced hyperglycemia, perioperative glucose control associated clinical outcome.
Conclusion
Among patients undergoing ascending aorta and aortic arch repair surgery, higher blood glucose levels before and after surgery are independent risk factors for in-hospital mortality, prolonged endotracheal intubation, multiple adverse clinical outcomes, and less likely to be discharged to home. Higher blood glucose is also associated with prolonged ICU LOS and hospital LOS. Further large-scale, well-designed prospective or clinical trials are required to elucidate the causality and confirm the optimal glycemic goal.
Funding
No funding was obtained for this study.
IRB Approval
HSC-MS-18-0371, on June 6, 2018.
References
- Zhou S, Yan SF, Sandhu HF, Choi WF, Mshvildadze MF. Perioperative hyperglycemia is an independent risk factor for surgical mortality in patients undergoing ascending aorta and arch repair. Anesthesia and Analgesia. Vol 132;2021:182-4.
- Lazar HL, McDonnell M, Chipkin SR, Furnary AP, Engelman RM, Sadha AR, et al. The society of thoracic surgeons practice guideline series: Blood glucose management during adult cardiac surgery. Ann Thorac Surg. 2009;87:663-9.
- Engelman DT, Ali WB, Williams JB, Perrault LP, Reddy VS, Arora RC, et al. Guidelines for perioperative care in cardiac surgery: Enhanced recovery after surgery society recommendations. JAMA Surg. 2019;154:755-766.
- Galindo RJ, Fayfman M, Umpierrez GE. Perioperative management of hyperglycemia and diabetes in cardiac surgery patients. Endocrinol Metab Clin North Am. 2018;47:203-222.
- Doenst T, Wijeysundera D, Karkouti K, Zechner C, Maganti M, Rao V, Borger MA. Hyperglycemia during cardiopulmonary bypass is an independent risk factor for mortality in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2005 Oct;130(4):1144.
- Vogt AP, Bally L. Perioperative glucose management: Current status and future directions. Best Pract Res Clin Anaesthesiol. 2020;34:213-224.
- Duggan EW, Carlson K, Umpierrez GE. Perioperative hyperglycemia management: An update. Anesthesiology. 2017;126:547-560.
- Jakob SM, Stanga Z. Perioperative metabolic changes in patients undergoing cardiac surgery. Nutrition. 2010;26:349-353.
- Wahba A, Milojevic M, Boer C, De Somer FM, Gudbjartsson T, van den Goor, J, et al. 2019 EACTS/EACTA/EBCP guidelines on cardiopulmonary bypass in adult cardiac surgery. Eur J Cardiothorac Surg. 2020;57:210-251.
- Hulst AH, Visscher MJ, Godfried MB, Thiel B, Gerritse BM, Scohy TV, et al. Liraglutide for perioperative management of hyperglycaemia in cardiac surgery patients: a multicentre randomized superiority trial. Diabetes Obes Metab. 2020;22:557-565.
- Ascione R, Rogers CA, Rajakaruna C, Angelini GD. Inadequate blood glucose control is associated with in-hospital mortality and morbidity in diabetic and nondiabetic patients undergoing cardiac surgery. Circulation. 2008;118:113-123.
- Thiele RH, Hucklenbruch C, Ma JZ, Colquhoun D, Zuo Z, Nemergut EC, et al. Admission hyperglycemia is associated with poor outcome after emergent coronary bypass grafting surgery. J Crit Care. 2015;30:1210-6.
- Polito A, Thiagarajan RR, Laussen PC, Gauvreau K, Agus MSD, Scheurer MA, et al. Association between intraoperative and early postoperative glucose levels and adverse outcomes after complex congenital heart surgery. Circulation. 2008;118:2235-2242.
- Li X, Zhou X, Wei J, Mo H, Lou H, Gong N, et al. Effects of glucose variability on short-term outcomes in non-diabetic patients after coronary artery bypass grafting: A retrospective observational study. Heart Lung Circ. 2019;28:1580-1586.
- Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355:773-778.
- Duncan AE. Hyperglycemia and perioperative glucose management. Curr Pharm Des. 2012;18:6195-6203.
- Oliver MF, Opie LH. Effects of glucose and fatty acids on myocardial ischaemia and arrhythmias. Lancet. 1994;343:155-158.
- Mjos OD. Effect of free fatty acids on myocardial function and oxygen consumption in intact dogs. J Clin Invest. 1971;50:1386-1389.
- Jarvela KM, Khan NK, Loisa EL, Sutinen JA, Laurikka JO, Khan JA. Hyperglycemic episodes are associated with postoperative infections after cardiac surgery. Scand J Surg. 2018;107:138-144.
- Martin ET, Kaye KS, Knott C, Nguyen H, Santarossa M, Evans R, et al. Diabetes and risk of surgical site infection: A systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2016;37:88-99.
- Gandhi GY, Nuttall GA, Abel MD, Mullany CJ, Schaff HV, O'Brien PC, et al. Intensive intraoperative insulin therapy versus conventional glucose management during cardiac surgery: a randomized trial. Ann Intern Med. 2007;146:233-243.
- Simha V, Shah P. Perioperative glucose control in patients with diabetes undergoing elective surgery. JAMA. 2019;321:399-400.
- Kawahito K, Sato H, Kadosaki M, Egawa A, Misawa Y. Spike in glucose levels after reperfusion during aortic surgery: assessment by continuous blood glucose monitoring using artificial endocrine pancreas. Gen Thorac Cardiovasc Surg. 2018 Mar;66(3):150-154.