Translational Perioperative and Pain Medicine (ISSN: 2330-4871)
ARTICLE DOI: 10.31480/2330-4871/147
Review Article | Volume 8 | Issue 4 Open Access
Ciprofol: A Novel Medication from Development towards Clinical Use
Tingting Wang, MD, PhD1, Shanglong Yao, MD, PhD1*, Limin Zhang, MD2 and Yong Liu, MS2
1Department of Anesthesiology, Institute of Anesthesiology and Critical Care Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
2Sichuan Haisco Pharmaceutical Group Co., Ltd., Chengdu, China
Shanglong Yao, MD, PhD, Department of Anesthesiology, Institute of Anesthesiology and Critical Care Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1277 Jiefang Avenue, Wuhan 430022, China, Tel: +862785351633, E-mail: ysltian@163.comEditor: Tianzuo Li, MD, PhD, Professor, Department of Anesthesiology, Beijing Shijitan Hospital Affiliated to Capital Medical University, Beijing, China, E-mail: trmzltz@126.com
Received: August 19, 2021 | Accepted: October 30, 2021 | Published: November 01, 2021
Citation: Wang T, Yao S, Zhang L, Liu Y. Ciprofol: A Novel Medication from Development towards Clinical Use. Transl Perioper & Pain Med 2021; 8(4):397-402.
Abstract
Ciprofol is a new medication for inducing anesthesia or sedation. According to the results of several clinical trials, ciprofol has a rapid onset, fast and complete functional recovery, and shares similar sedative effects and clinical characteristics with propofol. Propofol, as one of the most widely used anesthetics in clinical practice in China, is routinely used for clinical anesthesia, sedation for patients in intensive care unit, as well as for diagnostic and minor therapeutic procedures. However, propofol related adverse events such as respiratory depression, pain on injection site, and cardiovascular collapse are common. This review aims to explore the similarities and differences between ciprofol and propofol in their chemical structures, the mechanisms of anesthetic action and sedation, the similarities between the two medications found in various clinical trials, and the effect on the reduction in incidence of major adverse events. The review highlighted research and development of ciprofol and clinical evidence for the use of ciprofol in various clinical applications.
Keywords
Ciprofol, Propofol, Sedation/Anesthesia, Clinical applications
Introduction
Propofol, as a short-acting intravenous alkylphenol anesthetic, when administered, produces rapid and smooth anesthesia. Its working mechanism mainly relies on activation of the γ-aminobutyric acid-A (GABAA) receptor-chloride ion complex [1-3]. It inhibits the central nervous system pathways by increasing chloride ion conduction or desensitizing GABAA receptors [3,4]. Since its listing in 1986, it has drawn significant attention in clinical practice due to its rapid onset and recovery characteristics, high metabolic clearance and convenient target-controlled infusion. Thus, it has developed into a very commonly used intravenous anesthetic in clinical practice. However, it also has obvious limitations. Lowered blood pressure, Increased heart rate, Significant respiration inhibition, and Apnea are often reported when the propofol is induced under general anesthesia. Injection pain is also one of the most common adverse reactions elicited by propofol [5]. Therefore, in order to find new medications that can overcome these limitations and have the anesthetic effect similar to propofol, Haisco Pharmaceutical Group Co., Ltd. independently developed the new anesthetic medication HSK3486 (ciprofol) [6,7], which is expected to become a widely used anesthesia/sedation medication in clinical practice. This review summarizes the current relevant research literature and clinical trial information available globally, so as to facilitate the readers to understand its mechanism of action and clinical characteristics, and thereby provide the theoretical basis for its further use in clinical practice.
Structural Comparison of Ciprofol and Propofol
Propofol is marketed as Diprivan, which is chemically known as 2, 6-diisopropyl phenol. The working principle of propofol on the central nervous system is reported to be through the enhancement of GABAA receptor activity, which is affected in three ways [8]. A low concentration (2-100 μmol/L) of propofol can increase the whole-cell current induced by GABA, a medium concentration (100-2,000 μmol/L) directly activates the GABAA receptor, and a high concentration (> 2,000 μmol/L) produces non-competitive inhibitory effects on the GABAA receptor. In addition, propofol can be combined with two kinds of carriers, namely emulsions or cyclodextrin solutions. Some scholars believe that both carriers show similar patterns of anesthesia induction and maximum effects [9]. In 1986, AstraZeneca listed propofol injection with lipid emulsion as the carrier, which was approved and marketed in China in 1993, and the medication use rate reached 90%. However, pain on injection still occurs when propofol lipid emulsion is used. Rau, et al. [10] found that the number and intensity of pain were caused by long-chain triglyceride lipid emulsion, compared to the medium/long-chain triglyceride lipid emulsion, which was considered when preparing for the new medication ciprofol.
Ciprofol is a novel small molecule that is a GABAA receptor agonist. Its active ingredient, HSK3486, is a propofol analogue, which is a single diastereomer and contains an R-shaped hand center. Its chemical name is 2-[(1R)-1-cyclopropyl ethyl]-6-isopropyl-phenol. Since it has been proven that free propofol can bind with the inner wall of blood vessels and produce irritation and pain, we added medium/long-chain triglyceride lipid emulsion solvent to the ciprofol formulation to reduce the concentration of free ciprofol and thus reduce injection pain. As expected, previous animal studies demonstrated that ciprofol emulsion injection produces less pain than a propofol medium/long-chain triglyceride lipid emulsion.
Preclinical Animal Studies and Basic Pharmacology
Pharmacokinetics
Analysis of the disposition of ciprofol in animal study revealed a rapid clearance after intravenous administration, which indicated that it has a high plasma clearance rate. In addition, ciprofol reached a peak concentration rapidly and was widely distributed in the animal tissues after intravenous injection. Furthermore, it readily passes through the blood-brain barrier, which is beneficial for medication efficacy. After ciprofol was injected into Sprague-Dawley rats, it underwent extensive metabolism in plasma, urine, feces and bile. It was mainly preserved as the glucuronic acid conjugate of the protodrug (M4), monohydroxylated glucuronic acid conjugate (M5-1), and mono-oxidation metabolite (methyl oxidation to carboxylic acid, M2-3). The glucuronic acid conjugate excreted by bile underwent liver-intestinal recirculation and was finally excreted in feces in the forms of protodrug and hydroxylated metabolites. Ciprofol was shown to be mainly metabolized into mono-hydroxylated glucuronic acid conjugate (M5-1) and monohydroxylated sulfuric acid protodrug (M3) in dog plasma [6].
Pharmacodynamics
The pharmacodynamic results of animal experiments confirmed that the onset time, anesthesia time and time to ambulate after an intravenous injection of ciprofol lipid emulsion were similar to those of propofol. Dose-dependent anesthetic effects were also similar. However, the titer of ciprofol was five times that of propofol, with same rapid onset time and recovery profile (awake and ambulation time) (Table 1) [7].
Safety
The effectsof ciprofol on action potential, nervous system depressant, respiratory depression and cardiac Purkinje fibers were analyzed in various animal species. The results showed that the side-effects of ciprofol on the blood pressure and heart rate were less than that of propofol [6] (Figure 1).
Results of Multiple Clinical Trials
Phase 1 clinical trials
Phase 1 trials of ciprofol were performed in Australia [11] and China [12]. The phase 1a + b + c + d trials in Australia demonstrated that ciprofol was safe and well-tolerated by both healthy men and women at 0.128 to 0.810 mg/kg. The sedative and anesthetic onset and recovery times were rapid and in a dose-dependent manner. The dose of 0.540 mg/kg of ciprofol was equivalent to 2.5 mg/kg of propofol. The BIS peak reached a plateau at doses of 0.540 mg/kg or above, while a dose of 0.288 mg/kg group maintained moderate sedation and rapid recovery without any complications. Therefore, a dose of 0.288 mg/kg ciprofol is recognized to be suitable for inducing sedation/anesthesia in patients undergoing gastrointestinal endoscopic interventions. The 0.540 mg/kg group had maintained deeper sedation or general anesthesia, which is suitable to be used for more stimulating or longer operation times, such as minor gynecological surgery and orthopedic hip replacement. In addition, a phase 1d trial confirmed the safety and tolerability of ciprofol combined with etomidate.
The phase 1 China trial performed in China at 4 different doses (0.15 mg/kg, 0.40 mg/kg, 0.60 mg/kg and 0.90 mg/kg) demonstrated that the plasma concentration in healthy Chinese subjects peaked at 2-3 min, and decreased close to the baseline level after about 10 min. Metabolites were mainly excreted in the urine and the metabolic processes involved were not significantly different from propofol [7]. The results showed that the level and duration of sedation and anesthesia produced by ciprofol were dose-dependent, induction and rapid recovery, and the potency of ciprofol was 4-5 times that of propofol. Most of the adverse events (AEs) associated with ciprofol were mild and no severe ciprofol-related AEs occurred. No dose-dependent changes in the systolic blood pressure, diastolic blood pressure, or heart rate were observed in ciprofol treated subjects and were consistent with those found in the propofol group [12].
Clinical trials for various indications (phase 2 and phase 3)
Sedation and/or anesthesia for adult gastrointestinal endoscopy (phases 2a + 2b, 3): The safety and tolerance of ciprofol in the dose range of 0.15 mg/kg to 0.90 mg/kg were determined and confirmed in both phase 1 trials performed in China and Australia. On this basis, a phase 2(a + b) clinical trial was conducted to explore the maximum tolerated dose (MTD) and/or recommended dose (RP2D) of ciprofol for sedation/anesthesia in male or female patients undergoing selective diagnostic colonoscopy for less than 30 min [13]. It was also confirmed that 100% success rate of colonoscopy was achieved in patients with 0.2-0.5 mg/kg of ciprofol. The time of colonoscopy insertion for the 0.3-0.5 mg/kg ciprofol group was similar to that of the 1.0-2.0 mg/kg propofol group. In the 0.1-0.5 mg/kg ciprofol group, the time to recovery from the last medication administration was 11.1-16.4 min, which was longer than the time required to withdraw the colonoscope (5.5-10.7 min). Therefore, the anesthesiologists participating in this trial believed that using 0.3-0.5 mg/kg ciprofol had an overall better performance than propofol. The results showed a rapid onset, rapid recovery, less residual sedation, high satisfaction by physician, and safe/stable vital signs throughout the study period. The major AEs detected were hypotension, sinus bradycardia, twitching, tracheal obstruction, dizziness and injection pain. Hypotension and injection pain in the ciprofol group occurred much less frequently than in the propofol group. Hence, the recommended doses of 0.4 mg/kg and 0.5 mg/kg from phase 2a were used in phase 2b. In addition to the same colonoscopy success rate of 100% found in the phase 2a, clinical trial, it was well tolerated with mostly mild to moderate AEs occurring in the phase 2b. The AEs mainly involved respiratory depression and hypotension, which nevertheless met the needs of clinical colonoscopy.
Based on the data from the phase 2b clinical trial, efficacy and safety results were compared between the 0.4 mg/kg and 0.5 mg/kg groups. A dose of 0.4 mg/kg of ciprofol was selected to be used in a phase 3 study with a large sample scale. The non-inferiority comparison showed that the success rate of colonoscopy with ciprofol at 0.4 mg/kg was not inferior to that of propofol at 1.5 mg/kg, and without significant difference in cardiovascular system-related AEs. The results of this phase 3 clinical trial were sufficient to prove that ciprofol can reduce the discomfort of patients undergoing gastrointestinal endoscopy, increase patient's tolerance of endoscopy and the satisfaction of physicians and patients. There was little impact on respiratory depression, all of which ensured the safety of ciprofol use in patients to produce sedation/anesthesia for endoscopic diagnosis and treatment of various conditions (manuscript submitted).
In conclusion, the data of phase 2 and 3 clinical trials on sedation/anesthesia in adult endoscopy revealed that ciprofol has more advantages than propofol in alleviating patient discomfort and increased the tolerance and satisfaction of endoscopic procedures. In addition, the proportion of respiratory depression, apnea and hypoxia events in the ciprofol group was less than that in the propofol group. The influence on respiratory depression was also less than that in the propofol group. Changes in blood pressure and heart rate during the treatment were similar in the two groups. However, the incidence of medication-related AEs in the ciprofol group was significantly lower than that in the propofol group, which could better ensure the safety of patients during sedation/anesthesia for endoscopic diagnoses and treatment.
Induction of general anesthesia in adult surgery (phase 3): General anesthesia induces patients' loss of consciousness and can tolerate endotracheal intubation and other stimuli produced by surgery. Induction of anesthesia focuses on keeping the respiratory tract patent and not inhibiting cardiovascular functions. Phase 2 trial of general anesthesia induction using ciprofol in adults, based on the phase 1 trials performed in China and Australia, aimed to closely monitor any changes in the vital signs during anesthesia and prevent and control the risks of ciprofol related adverse reactions such as hypotension and respiratory depression. In the phase 2 trial, the enrolled subjects were hospitalized patients who needed endotracheal intubation under general anesthesia for ≤ 3 h for elective surgery, without emergency, cardiothoracic or brain operations. The inclusion criteria included ASA grade I-II; Age ≥ 18 and < 65-years-old; Population with body mass index [BMI] ≥ 18 ≤ 30 kg/m2 (NCT). In the phase 3 trial, the same kind of subjects were selected, and a multicenter, randomized, double-blind, parallel controlled was designed based on the phase 2 findings. The initial dose of ciprofol was 0.4 mg/kg and an additional top-up dose of 0.2 mg/kg if required. The results showed that the success rate of anesthesia induction in the ciprofol and propofol groups was 100%, and the 95% CI of the success rate difference was (-4.18%, 4.18%). The results of the secondary endpoints in the ciprofol and propofol groups were similar, indicating that induction was rapid and the required time virtually identical. The average BIS score first exhibited a downward trend after administration, then an upward trend, and was relatively stable during anesthesia maintenance. The mean anesthesia induction satisfaction was also comparable between the ciprofol and propofol groups. There were no TEAEs (Treatment Emergent Adverse Event) in both groups that led to withdrawal and no significant difference in the incidence of TEAE related to the study medications and SAE between the ciprofol and propofol groups (P > 0.05). The incidence of injection pain in the ciprofol group was significantly lower than that in the propofol group (6.8% vs. 20.5%, P < 0.05), and the incidence of cardiovascular TEAEs (including hypotension, bradycardia, elevated blood pressure and tachycardia) was also lower than that in the propofol group.
The results of the above phase 2 and phase 3 clinical trials demonstrated that ciprofol had a rapid onset in general anesthesia induction. The 100% induction success rate, and a successful induction duration comparable to that of propofol. Moreover, the dosage of ciprofol required was lower, and the general anesthesia ability of 0.4 mg/kg ciprofol was comparable to that of 2.0 mg/kg propofol, with fewer cardiovascular AEs and less injection pain. Therefore, the efficacy and safety of ciprofol are both excellent. All the data support the use of ciprofol as a new medication for general anesthesia induction of surgical patients and has now been approved for marketing.
Sedation and/or anesthesia in bronchofibroscopy (phase 3): Bronchoscopy is the most common clinical procedure used by chest physicians. Most of the patients experience much discomfort and complications caused by fear, pain, respiratory distress and nasopharyngeal irritation [14]. Therefore, patients are usually moderately sedated using superficial anesthesia, analgesia and sedatives during bronchofibroscopy, so that they are placed in the optimal operating environment. Currently, propofol is commonly used to induce sedation, produce amnesia, with the added advantage of a shorter recovery time. Therefore, a multicenter, double-blind, randomized, non-inferiority, parallel phase 3 study has assessed the efficacy and adverse effects of ciprofol in patients undergoing bronchoscopy with propofol as the control group. Patients were randomized to receive 0.4 mg/kg ciprofol or 2.0 mg/kg propofol in a ratio of 1:1. Bronchofibroscopy was performed in both groups and no patients received alternative sedation/anesthesia, with the success rate in both groups being 100%. The time to complete consciousness (8.53 min vs. 6.71 min) and discharge time (13.17 min vs. 10.74 min) in the ciprofol group was slightly longer than that in the propofol group (all P < 0.05). The incidence of AEs in the ciprofol group was lower than that in the propofol group (52.6% vs. 76.5%, P < 0.05). The incidence of injection pain was also significantly lower in the ciprofol group than the propofol group (4.4% vs. 39.4%, P < 0.001). The pharmacokinetics properties of ciprofol and propofol were found to be very similar. This clinical trial demonstrated the efficacy and safety of ciprofol in bronchoscopy and had the significant advantage of reducing the incidence of injection pain.
Sedation in adults in ICUs (phase 2 and phase 3): In addition to propofol, benzodiazepines are the most widely used sedative medications in ICUs in China, which include midazolam, lorazepam and diazepam. Sedative medications should ideally be administered by continuous intravenous infusion. A loading dose should be given first to reach the sedation target as soon as possible (common doses are shown in Table 2). Diazepam and midazolam are known to produce rapid sedation and can be used in patients with acute agitation, and are suitable for assisted ventilation therapy where endotracheal intubation has been established. Their use in patients who have not yet been intubated may result in respiratory depression. For short-term sedation of < 3 days, propofol and midazolam produced similar clinical effects. The time to consciousness and the extubation time with propofol was significantly lower than that for midazolam treated patients but did not affect a patient's stay in the ICU. The effect of lorazepam is slow with a long clearance time and it is easy to produce oversedation. Therefore, propofol and midazolam are preferred for short-term sedation in ICU patients. For long-term sedation over 3 days, propofol was associated with faster recovery and earlier extubation than midazolam. However, propofol was more prone to produce hypotension during the induction period, while midazolam was more prone to elicit respiratory depression. Dexmedetomidine is a central α2-adrenoceptor agonist; it has strong sedative and anti-anxiety effects and can be used for postoperative analgesia and sedation without the adverse reactions of respiratory depression produced by morphine. It has a short half-life (2 h) and produces good short-term and long-term sedation. However, it is expensive and has not been widely used in the ICU. The continued use of dexmedetomidine can cause bradycardia, hypotension and other AEs. We anticipate that ciprofol will have desirable effects in the ICU. A phase 2 multicenter, open-label, randomized, ciprofol-positive controlled ICU clinical trial has been completed. The initial loading dose of ciprofol was 0.1-0.2 mg/kg (0.5-5 min), and the maintenance dose 0.3 mg/kg/h, which could be adjusted between 0.06-0.8 mg/kg/h. The median time required for sedation to reach the target effect of -2 – +1 RAAS within 6-24 h of administration was 60.0 min, which is not different from propofol. Most of the AEs in the two groups were grade 1 or 2. AEs related to the medication and sedation were significantly lower in the ciprofol group compared to the propofol group, including hypotension (7.7% vs. 30.8%) and sinus bradycardia (3.8% vs. 7.7%). It is noteworthy that the plasma concentration-time curve of ciprofol was similar to that of propofol. Phase 3 studies are ongoing in the patient inclusion phase [15].
Current research status and progress of ciprofol
As a new intravenous anesthetic, through several preclinical animal experiments and clinical phase 1, 2, 3 trials as well as clinical trials with a variety of indications, it has been unequivocally established that ciprofol lipid emulsion injection has excellent pharmacological properties and pharmacokinetic characteristics, and is safe and reliable to use in clinical practice. Ciprofol can be used for sedation and/or anesthesia during gastrointestinal endoscopy diagnosis and treatments. It will achieve stronger anesthesia depth, a shorter onset time, rapid recovery rate and fewer side effects than propofol and meet clinical requirements better. During the induction of anesthesia, ciprofol also showed good efficacy and safety, has broad clinical application prospects. Meanwhile, ciprofol was safely tolerated with prolonged infusion such as anesthesia maintenance and ICU sedation. In general, ciprofol plays a similar role to propofol, an intravenous anesthetic commonly used in clinical practice, and is superior to propofol in the incidence of injection pain, respiratory and cardiovascular adverse events. Given the limitations and shortcomings of the general anesthetics currently used and the vast share of propofol in the anesthetics market, it can predict that ciprofol lipid emulsion injection has the potential to become an alternative intravenous anesthetic after propofol (medium/long-chain fat emulsion). Ciprofol, with its unique advantages, can better serve clinical practice, and benefit patients.
Conflict of Interests
Limin Zhang and Yong Liu are employees of Sichuan Haisco Pharmaceutical Group Co., Ltd. The other authors declare no conflicts of interest.
Funding
The study was supported by Major Technical Innovation Special Project of Hubei Province (grant number 2019ACA167).
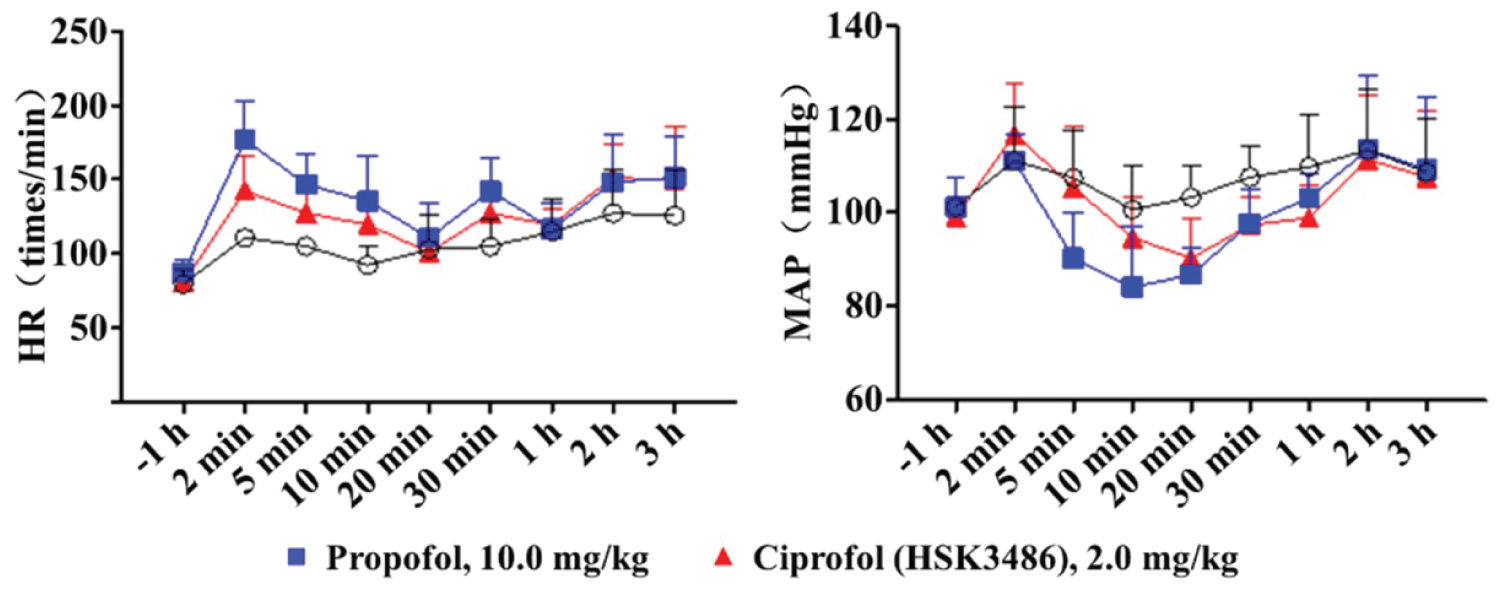
Figure 1: Comparison of cardiovascular safety between ciprofol (HSK3486) and propofol.
Table 1: Chemical structure diagram of ciprofol and propofol.
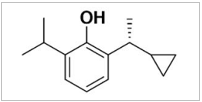 Ciprofol (HSK3486) |
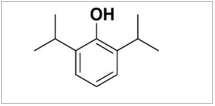 Propofol |
|
|
ED50 LD50 TI (LD50/ED50) SI (LD5/ED95) GABA binding assay (10 μM) (inhibition%) Safe and effective dose range |
1.47 mg/kg 9.86 mg/kg 6.7 4.1 85 2-8 mg/kg |
11.65 mg/kg 31.26 mg/kg 2.7 1.5 10 14-20 mg/kg |
Table 2: Comparison of commonly used sedatives/anesthetics in the adult ICU.
| Drugs | Feature | Administration | Adverse event |
|
Propofol Ciprofol |
Most common, rapid onset Phase 3 clinical trial |
Loading doses: 1-3 mg/kg; Recommended |
Blood pressure drop and sinus bradycardia Injection pain, Lipid infusion syndrome Blood pressure drop and |
References
- Kay B, Rolly G. I.C.I. 35868, a new intravenous induction agent. Acta Anaesthesiol Belg. 1977; 28(4): 303-16.
- Trapani G, Altomare C, Liso G, Sanna E, Biggio G. Propofol in anesthesia. Mechanism of action, structure-activity relationships, and drug delivery. Curr Med Chem. Feb 2000; 7(2): 249-71. doi:10.2174/0929867003375335
- Ito Y, Izumi H, Sato M, Karita K, Iwatsuki N. Suppression of parasympathetic reflex vasodilatation in the lower lip of the cat by isoflurane, propofol, ketamine and pentobarbital: implications for mechanisms underlying the production of anaesthesia. Br J Anaesth. Oct 1998; 81(4): 563-8. doi:10.1093/bja/81.4.563
- Murphy PG, Myers DS, Davies MJ, Webster NR, Jones JG. The antioxidant potential of propofol (2,6-diisopropylphenol). Br J Anaesth. Jun 1992; 68(6): 613-8. doi:10.1093/bja/68.6.613
- Doenicke AW, Roizen MF, Rau J, et al. Pharmacokinetics and pharmacodynamics of propofol in a new solvent. Anesth Analg. Dec 1997; 85(6): 1399-403. doi:10.1097/00000539-199712000-00040
- Qin L, Ren L, Wan S, et al. Design, Synthesis, and Evaluation of Novel 2,6-Disubstituted Phenol Derivatives as General Anesthetics. J Med Chem. May 11 2017; 60(9): 3606-3617. doi:10.1021/acs.jmedchem.7b00254
- Bian Y, Zhang H, Ma S, et al. Mass balance, pharmacokinetics and pharmacodynamics of intravenous HSK3486, a novel anaesthetic, administered to healthy subjects. Br J Clin Pharmacol. Jan 2021; 87(1): 93-105. doi:10.1111/bcp.14363
- Hara M, Kai Y, Ikemoto Y. Enhancement by propofol of the gamma-aminobutyric acidA response in dissociated hippocampal pyramidal neurons of the rat. Anesthesiology. Oct 1994; 81(4): 988-94. doi:10.1097/00000542-199410000-00026
- Johnson KB, Egan TD, Layman J, Kern SE, White JL, McJames SW. The influence of hemorrhagic shock on etomidate: a pharmacokinetic and pharmacodynamic analysis. Anesth Analg. May 2003; 96(5): 1360-8, table of contents. doi:10.1213/01.ane.0000055804.30509.69
- Rau J, Roizen MF, Doenicke AW, O'Connor MF, Strohschneider U. Propofol in an emulsion of long- and medium-chain triglycerides: the effect on pain. Anesth Analg. Aug 2001; 93(2): 382-4 , 3rd contents page. doi:10.1097/00000539-200108000-00029
- Ludbrook G, Li F, Sleigh J, Liang Y. Assessments of Onset and Duration of Drug Effects and Pharmacokinetics by Dose Level of HSK3486, a New Sedative-Hypnotic Agent, in Healthy Female/Male Subjects: A Phase I Multiarm Randomized Controlled Clinical Trial. Anesth Analg. Jan 15 2021; doi:10.1213/ane.0000000000005343
- Teng Y, Ou M, Wang X, et al. Pharmacokinetic and pharmacodynamic properties of ciprofol emulsion in Chinese subjects: A single center, open-label, single-arm dose-escalation phase 1 study (In press). Am J Transl Res 2021; 13(9):XXX-XXX.
- Teng Y, Ou M, Wang X, et al. Efficacy and safety of ciprofol for the sedation/anesthesia in patients undergoing colonoscopy: Phase IIa and IIb multi-center clinical trials. Eur J Pharm Sci. Sep 1 2021; 164: 105904. doi:10.1016/j.ejps.2021.105904
- Yamamoto S, Igarashi T, Tetsuka K, Endo S. Bispectral index monitoring of midazolam sedation during flexible bronchoscopy. J Bronchology Interv Pulmonol. Oct 2009; 16(4): 241-4. doi: 10.1097/LBR.0b013e3181bb781f
- Liu Y, Chen C, Liu N, et al. Efficacy and safety of ciprofol sedation in ICU patients with mechanical ventilation: a clinical trial study protocol. Adv Ther. 2021Oct; 38(10): 5412-5423. doi: 10.1007/s12325-021-01877-6