Translational Perioperative and Pain Medicine (ISSN: 2330-4871)
ARTICLE DOI: 10.31480/2330-4871/146
Original Research | Volume 8 | Issue 4 Open Access
Long-Term Effects of Critical Care Insults on Lipoprotein Metabolism
Krzysztof Laudanski1,2*, Jihane Hajj3, Ciara Riedel4, Da Liu5, Josh Maret6, Mariana Restrepo7 and Kumal Siddiq8
1Assistant Professor, Department of Anesthesiology and Critical Care, Hospital of Pennsylvania, Philadelphia, PA, USA
2Senior Fellow, Leonard Davis Institute of Health Economics, The University of Pennsylvania, Philadelphia, PA, USA
3Assistant Professor, Department of Nursing, Widener University, Philadelphia, PA, USA
4Graduate Student, School of Nursing, The University of Pennsylvania, Philadelphia, PA, USA
5Assistant Professor, Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang, People Republic of China
6Drexel University College of Medicine, Philadelphia, PA, USA
7Undergraduate Student, College of Arts and Sciences, The University of Pennsylvania, Philadelphia, PA, USA
8Undergraduate Student, College of Arts and Sciences, Drexel University, Philadelphia, PA, USA
Krzysztof Laudanski, Assistant Professor, Department of Anesthesiology and Critical Care. Department of Neurology, Senior Fellow, Leonard Davis Institute for Healthcare Economics, JMB 127, 3620 Hamilton Walk, Philadelphia, PA 19146, USA, E-mail: klaudanski@gmail.comEditor: Yuan-Xiang Tao, Ph.D., M.D., Professor and Vice Chair, Director, Center for Pain Medicine Research, Department of Anesthesiology, Editor in Chief, The Translational Perioperative and Pain Medicine, Rutgers, The State University of New Jersey, New Jersey Medical School, 185 S. Orange Ave., MSB, E-661, Newark, NJ 07103, USA, Tel: 973-972-9812, E-mail: yt211@njms.rutgers.edu
Received: August 16, 2021 | Accepted: October 08, 2021 | Published: October 13, 2021
Citation: Laudanski K, Hajj J, Riedel C, Liu D, Maret J, et al. Long-Term Effects of Critical Care Insults on Lipoprotein Metabolism. Transl Perioper & Pain Med 2021; 8(4):385-396
Abstract
Background: Several reports demonstrated acute changes in lipoprotein profiles during the acute phase of critical care illness (CCI), with significantly fewer investigations suggesting the persistence of lipid abnormalities past 28 days from onset of CCI, or into recovery. Theoretically, their persistence into a recovery of CCI may profoundly impact health as dyslipidemias are considered critically important in the natural history of atherosclerosis. Considering the increased incidence of survivorship after CCI (sepsis, trauma, severe surgery, burn), and the profound effect of even small changes in lipid makeup over time, investigating the persistence of lipid profile abnormalities addresses important health concerns. Here, we conducted a comprehensive review focusing on long-term (28 days or greater) lipoprotein abnormalities after CCI onset.
Methods: The supervised, narrative literature review utilized the PubMed, EBSCO, and Google Scholar databases using keywords (sepsis, trauma, surgery, stroke, recovery). In addition, we pursued studies focusing on 28 days observation periods, at minimum, after the resolution of acute inflammation. We identified 26 studies fitting our criteria, with another 11 included because of their input value.
Results: Only a handful of studies investigated long-term lipid profiles after sepsis, trauma, surgery, or traumatic brain injury. Persistent depression of HDL-c and LDL-c serum levels were the hallmarks of several post-CCI conditions. Furthermore, few investigators reported an increase in the oxidation of LDL. One animal study linked these observations to acceleration in the atherosclerotic process in animals surviving weeks after sepsis resolution.
Conclusions: Scant data indicates that recovery from critical care illness is associated with several persistent lipoprotein abnormalities. Numerous gaps in knowledge persist, particularly in terms of the clinical translation of observed lipid abnormalities into long-term cardiovascular outcomes post-CCI.
Keywords
LDL-c, HDL-c, VLDL-c, Oxidized LDL-c, Cholesterol, Sepsis, Trauma, Traumatic brain injury, Surgery, Cerebrovascular accident, Lipoprotein profile
Abbreviations
ACS: Acute Coronary Syndrome; AIS: Acute Ischemic Stroke; APACHE: Acute Physiology, Age, and Chronic Health Evaluation; ApoA-I: Apolipoprotein A-I; ApoB: Apoliprotein B; ASCVD: Atherosclerotic Cardiovascular Disease; CABG: Coronary Artery Bypass Grafting; CCI: Critical Care Illness; CETP; Cholesteryl Ester Transfer Protein; HDL-C: High Density Lipoprotein Cholesterol; ICU: Intensive Care Unit; LDL-C: Low Density Lipoprotein Cholesterol; MICU: Medical Intensive Care Unit; MOD: Multiple Organ Dysfunction; OxLDL: Oxidized Low Density Lipoprotein; PLTP: Phospholipoproteins Transfer Protein; MI: Myocardial Infarction; sdLDL: Small Dense Low Density Lipoprotein; SIRS: Severe Inflammatory Response Syndrome; SN1: Substitution Nucleophilic Unimolecular; STEMI: ST-Elevation Myocardial Infarction; TBI: Traumatic Brain Injury
Background
Among survivors of critical care illness (CCI), the acute inflammatory response determines the trajectory of recovery [1-4]. Interestingly, CCI survivors acquire new allostasis leading to alterations in homeostasis with unclear, long-term health consequences [5-8]. One of the features of newly emerged allostasis is immune system and metabolic re-programming. Considering the essential role of the immune system and metabolome in responses to CCI and the progression of atherosclerosis, one could hypothesize that the immune system's atypical post-CCI performance may significantly impact the natural evolvement of atherosclerosis. Furthermore, acute illness is associated with changes in the lipoprotein profile during acute phase, endothelial injury and free radical environment. All of them may have an unfavorable effect on atherosclerosis progression setting the stage for long-term abnormalities during acute phase of illness [9,10]. Their persistence into recovery further increases the risk of atherosclerotic reactions. Noteworthy, even minor alterations in lipid metabolism affecting atherosclerotic plaque development have the potential to compound after years, especially if other processes accelerating atherosclerosis (diabetes, low grade inflammation) are already present [11].
Under nominal conditions, low-density lipoprotein cholesterol (LDL-c) plays an essential role in determining the progression of atherosclerosis. Significantly more pro-atherogenic forms such as oxidized LDL-c (oxLDL-c) are particularly prevalent in diabetes, obesity, and chronic inflammation [12,13]. Other pivotal processes contributing to atherosclerosis are endothelial dysfunction and infiltration of inflammatory leukocytes, which are both heavily driven by inflammation and oxidative stress [14]. All these conditions are prevalent during critical care illness and may persist afterwards, leading to accelerated atherosclerosis [15,16]. Furthermore, one needs to keep in mind that pre-existing lipoprotein profiles impact the progression of critical illness and set the stage for any atherosclerotic profile before CCI [17-20]. However, there is a gap of knowledge regarding the natural history of lipoprotein profile recovery long-term after CCI [21,22]. Considering the rapidly increasing population of sepsis survivors, the abnormalities of lipoproteins post-inflammatory states may provide insight into the long-term cardiovascular consequence of CCI [23].
The purpose of this review is to explore the effects of CCI on components of the lipoprotein profile during the recovery phase of these illnesses. We focused on sepsis, trauma, surgery, and traumatic brain injury due to their prevalence and high incidence of prolonged recovery among survivors [24-26]. We included literature on chronic liver failure considering the critical role of the liver in lipoprotein metabolism. Lastly, we investigated the recovery from cerebrovascular accidents and acute coronary syndrome because lipoproteins are particularly pivotal for the progression of these illnesses and the trajectory of both disease processes are frequently complicated by CCI. In both conditions, surgery is frequently implemented to correct pathological problem or address emerging complications. There is no uniform, pre-existing definition to differentiate acute and chronic changes in CCI, therefore, we decided to use the definition of greater or equal to 28 days as the landmark for post-CCI period. There are several manuscripts describing the abnormalities of lipoprotein metabolism during acute diseases, but we focused on the less explored, long-term aspects of dyslipidemias which are potentially more consequential for health maintenance [10,15,21,27]. We focused on situation where the pathological process has resolved so the chronic conditions (malaria, HIV, chronic hepatitis) were not included here. Considering significant differences in human and animal immunological mechanisms, we attempted to avoid animal studies when able. Due to the lack of data on this specific topic, some animal studies were included [28-30]. This review combines the limited long-term data with theoretical implications of investigations focusing on the short-term outcomes to provide a discussion frame. The novelty of this narrative review stems from its sole focus on the effects of common CCI on long-term aberrations in the lipoprotein profile with a particular emphasis on the acceleration of the atherosclerotic process.
Materials and Methods
This comprehensive review of the literature was completed utilizing the PubMed, EBSCO, and Google Scholar databases (Figure 1; Supplement #1). The following keywords, using MESH terms when possible, were utilized: Sepsis (C01.757), trauma injuries (Q000293), liver failure (C06.552.308.500), myocardial ischemia (C14.280.647), stroke (C10.228.140.300.775), surgery (C14.907.253.855), traumatic brain injury (C10.228.140.199.444, C10.900.300.087.235, C26.915.300.200.194), critical care (E02.760.190), postoperative care (E04.614.750), postoperative complication (C23.550.767), atherosclerosis (C14.907.137.126.307), lipoproteins (D10.532), survivors (M01.860), inflammation, critical care outcomes, treatment outcome, and long-term, identifying a total of 13,953 records. After removal of duplicates 12,726 records were identified. Only adult human investigations were included, except for a few animal studies where we had the ability to reach out directly to the investigators. The search criteria included full-text original studies (case report, journal article, clinical trials, clinical study, multicenter, and observational studies) which were written in English, have an abstract, and were published after 2000 with total of 9,946 records demonstrated. We screened the collected references resulting in the elimination of 9,981 articles due to the following reasons: 1) Irrelevance to the search objective, 2) The nature of the articles being case reports, and 3) System misclassification. A total of 55 full-text articles were closely evaluated, of which 29 articles were eliminated from this review for the following reasons: 1) Irrelevant study objectives, 2) Irrelevant results or outcomes, 3) Incorrect timing or stage of the disease, 4) Misclassification in type of article (case reports, case series report), and 5) Unpublished research methodology. A total of 26 original articles were included in this review. An additional 11 articles were included after following the leads from reviewed articles and suggestions during the review process. One manuscript denoting an animal study was included as well. Table 1 described the key human studies.
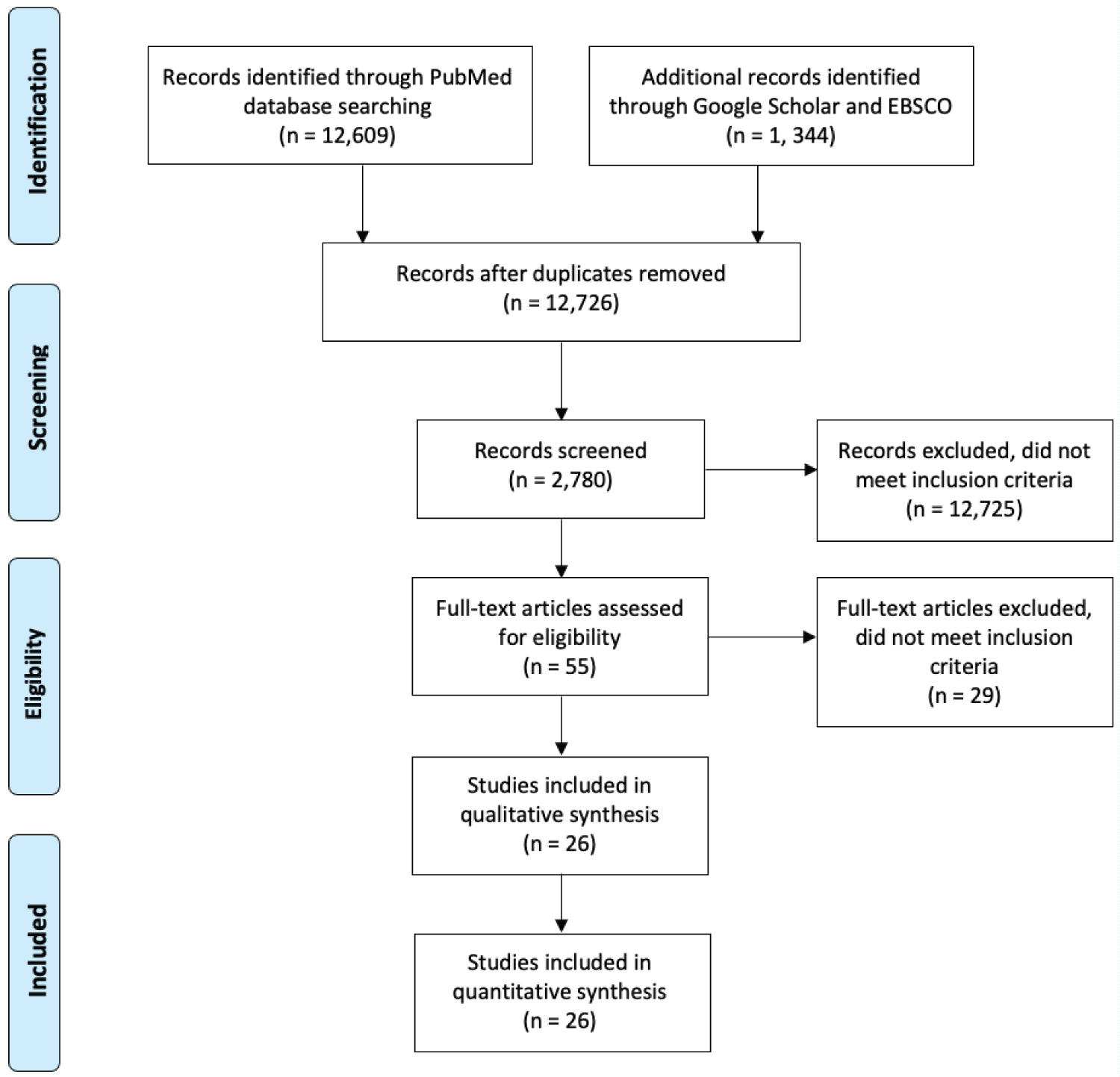
Figure 1: PRISMA Diagram: The literature included in this review was selected using the following criteria.
Table 1: Review of existing literature devoted to post-acute illness changes in critical care illnesses.
| Ref# | Primary Author | Design | Sample (n) |
Observational Period | Findings |
|---|---|---|---|---|---|
|
Ref# |
Primary Author |
Design |
Sample
(n) |
Observational Period |
Findings |
|
[8] |
Hacquebard, M. |
Prospective Study |
21 |
2 days |
Decrease in LDL and but only moderate decrease in HDL |
|
[14] |
Hossain, E. |
Experimental Study |
> 10 × 104 |
Unknown |
LPS enhances the cellular uptake of oxLDL in macrophages by upregulating LOX-1 through activation of ERK signaling pathway. |
|
[16] |
Kaynar, A. M |
Prospective, Randomized Animal Study |
78 |
N/A |
Cecal ligation & puncture accelerates atheroma development (46% by 3 months, p = 0.03) |
|
[17] |
Kaysen G. A. |
Retrospective Cohort Study |
37,250 |
January 1, 2000 - December 31, 2012 |
Lipoproteins levels are inversely associated with infectious and all- cause mortality in the fully adjusted model (hazard ratio: 0.83; 95% CI: 0.75-0.91.) |
|
[18] |
Iribarren, C. |
Cohort Study |
120,571 |
1979-1993 |
Possible inverse association between the incidence of infections and total cholesterol. |
|
[19] |
Feng, Q. |
Cohort Study |
61,502 |
January 1, 1993 - December 31, 2017 |
The risk of sepsis and poor outcomes do not seem to be directly altered due to levels of LDL-C (r = 0.24; P < 2.2 × 10−16). |
|
[20] |
Chien, Y. - F. |
Prospective Study |
40 |
November 2006 - January 2009 |
Decrease serum HDL cholesterol levels may be a prognostic value in the first seven days (8.5 vs. - 17.4 mg/dL, P = 0.04). |
|
[22] |
Lee, S. H. |
Retrospective Study |
926 |
January 2007 - December 2015 |
Severe persistent hypocholesterolemia independently predicted in- hospital mortality (HR, 3.961; 95% CI). |
|
[29] |
Szeto, C. C. |
Cross- Sectional Study |
30 |
Unknown |
Endotoxemia may have a role in a systemic inflammatory state and accelerated atherosclerosis in PD patients. |
|
[30] |
Lüthold, S. |
Observational Study |
101 |
September 1996 - June 1997 |
HDL-C and TC levels are lower in infected than in non-infected critically ill patients (p < 0.001). |
|
[31] |
Fraunberger, P. |
Letter to the Editor |
-- |
N/A |
Hypocholesterolemia could be linked with mortality in inflammation during critical illness. |
|
[33] |
Nishida, M. |
Cross- Sectional Study |
1078 |
2004 |
Components of MetS present profound effects on subclinical atherosclerosis in women rather than in men. |
|
[32] |
Al-Banna, N. |
Review |
-- |
N/A |
OxLDL and LOX-1 are linked to proinflammatory disease mechanisms. |
|
[34] |
Lara- Guzman, O. J. |
Experimental Study |
35 |
Unknown |
Induction biomarkers linked to oxidative stress and inflammation are triggered when THP-1 macrophages were treated with oxLDL (p < 0.001). |
|
[29] |
Wolfe, R. R. |
Experimental Animal Study |
11 |
N/A |
The increase in triglyceride concentration in fasting dogs with gram-negative sepsis is the result of an increase in VLDL production (5-fold increase). |
|
[35] |
Tanaka, S. |
Prospective Observational Study |
40 |
Unknown |
HDL levels are dramatically decreased in the acute phase of septic shock and there is a shift toward large HDL particles (r = 0.39, p = 0.012). |
|
[31] |
Lekkou, A. |
Prospective Study |
50 |
Unknown |
Low cholesterol and lipoprotein concentrations are detected in septic patients, especially in individuals with poor outcome (HDL-C, p < 0.05). |
|
[32] |
Randomized Controlled Trial |
20 |
Unknown |
Inflammation modulates human HDL composition (reduced HDL phospholipoproteins ~25%) and function in vivo. |
|
|
[33] |
Grion, C. M. |
Prospective Cohort Study and Case- Control Analysis |
1719 |
May 31st - December 1st, 2005 |
Each 1 mg dL (-1) increase in HDL decreased the odds of severe sepsis by 3% during hospitalization. |
|
[34] |
Wu, Q. |
Retrospective Study |
99 |
January 2017 - December 2017 |
The TG elevation that occurred during ICU stay was associated with worse outcomes and long- term hospitalization of the ICU. |
|
[35] |
Cirstea, M. |
Blinded, Observational Cohort Study |
200 |
January 2011 - June 2014 |
Plasma HDL-C level was the best prognostic marker for adverse outcomes in a suspected sepsis cohort (p = 0.469). |
|
[36] |
Larsen, S.F. |
Prospective Cohort Study |
2463 |
N/A |
Increased incidence of cerebrovascular demonstrated by 3 patients. |
|
[37] |
Milone, M. |
Observational, Comparative Cohort Study |
160 |
Unknown |
SG and MGB showed a similar efficacy in the improvement of lipoproteins profile of obese patients after 12-month follow-up (p = 0.039). |
|
[39] |
Genua, I. |
Retrospective, Observational Study |
185 |
January 2007-March 2015 |
The maximum increase in postoperative HDLc concentrations is observed 2 years after surgery (26.2% increase from baseline; p = 0.000). |
|
[44] |
Gordon, B. R. |
Consecutive, Prospective Case Series |
111 |
Unknown |
Low cholesterol and lipoprotein concentrations found in critically ill surgical patients correlate with interleukin-6, soluble interleukin- 2 receptor, and interleukin-10 concentrations and predict clinical outcomes (association between IL-6 and apolipoprotein A-I, p < 0.001). |
|
[42] |
Dunham, C. M. |
Cohort Study |
28 |
November 2000 - October 2002 |
Convalescing patients have improved cholesterol levels (143 ± 35), whereas dying patients appear to have progressive hypocholesterolemia (117 ± 27 mg/dl.) |
|
[44] |
Akan, A. A. |
Experimental Animal Study |
40 |
N/A |
The spleen may have an important effect on lipoproteins metabolism and splenic auto transplantation may be protective in conditions with increased lipoproteins levels (HDL increased post-operatively, p < 0.05). |
|
[45] |
Li, Y. |
Retrospective Matched Cohort Study |
130 |
1990-2015 |
Splenectomy is associated with lower coronary artery atherosclerotic plaque severity (p = 0.03) and altered coronary artery macrophage distribution (p ≤ 0.0002). |
|
[46] |
Robinette, C. D. |
Comparative Study |
740 |
1939-1945 |
Risk of fatal infections is increased by asplenia; however, the risk of cancer was not increased. |
|
[47] |
Ferrante, A. |
Retrospective Study |
24 |
Unknown |
Changes in proportion of regulatory T cells and monocytes may play a part in depressed mitogen responses of MNL from splenectomized subjects (P < 0.001). |
|
[48] |
Abbott, R. D. |
Comparative Cohort Study |
2425 |
1969-1971 |
Screening for total cholesterol alone in men and women aged 50 and older may not adequately identify the coronary candidate. |
|
[49] |
Kumar, P. |
Prospective evaluation |
60 |
N/A |
Most patients had high LDL-C and low HDL-c levels within 24 hours of myocardial infarction. |
|
[50] |
Thakkar, H. |
Case Control Study |
260 |
Unknown |
Acute Coronary Syndrome is associated with reduced HDL functions (p < 0.001). |
|
[51] |
Górecki, A. |
Observational Clinical Study |
348 |
Unknown |
Higher levels of total cholesterol and LDL cholesterol during first 24 hours of acute myocardial infarction have a strong negative prognostic value. |
|
[52] |
Hacquebard, M. |
Cohort Study |
20 |
Unknown |
Reduced α-toc level observed in the LDL fraction after cardiac surgery is due to the reduced circulating LDL particles (p < 0.0001). |
|
[53] |
Hlatky, M. A. |
Comparative Cohort Study |
23,353 |
January 2000 - December 2007 |
Patients who received CABG were less likely than patients who received PCI to fill prescriptions for secondary preventive medications and to use those medications consistently in the first year after the procedure (p < 0.0001). |
|
[54] |
Trieb, M. |
Cross Sectional Study |
59 |
Unknown |
Liver disease alters cholesterol efflux capacity, paraoxonase activity, anti-inflammatory and endothelial regenerative activities of apoB-depleted serum. |
|
[56] |
Giovannini, I. |
Prospective Study |
92 |
N/A |
Hypocholesterolemia in postoperative and critically ill patients is a cumulative index of severity of illness and has a relationship with poor prognosis (p < 0.001). |
|
[57] |
Zeljkovic, A. |
Prospective Study |
362 |
N/A |
sdLDL is an independent predictor of both AIS onset (p < 0.001) and consecutive short-term mortality (p < 0.05). |
|
[58] |
Perovic, E. |
Cohort Study |
52 |
2011-2012 |
Early after the onset of acute ischemic stroke there is a marked change in the concentration of serum lipoproteins. |
|
[59] |
Wang, J. |
Randomized Animal Study |
15 |
N/A |
Progression of atherosclerosis is accelerated following traumatic brain injury (p < 0.05). |
|
[60] |
Ahmadi, N. |
Prospective Cohort Study |
543 |
2007-2009 |
Mild traumatic brain injury is associated with the severity of coronary atherosclerosis. |
|
[61] |
Venetsanou, K. |
Comparative Cohort Study |
75 |
Unknown |
LDL alone (lower, p = 0.003) or with IL-6 & IL-8 (higher, p < 0.0001) could be a prognostic factor of 30-day mortality for patients with traumatic brain injury. |
Results
Sepsis and other acute inflammatory conditions
Sepsis results in significant inflammation and immunological re-programming, potentially affecting the lipoprotein profile short- and long-term [1,6]. During an acute sepsis episode, the endothelium thickens while monocytes become inflammatory with frequent emergence of atypical, pro-atherogenic M2 type [28]. HDL-c and LDL-c levels were almost uniformly depressed proportionally to the severity of sepsis, while the oxidation of LDL-c (ox-LDL-c) was increased during the acute phase sepsis [21,29-36]. The serum VLDL-c level was increased during the acute illness, but it is unclear how long this effect persist [37]. The components of more advanced parts of the lipogram were not studied, with the exception for ApoA-1, which is depressed during the sepsis episode [30,31]. This data strongly suggests that sepsis could accelerate atherosclerosis due to the lost HDL-c protection, especially if this depletion change persists. Unfortunately, a robust body of evidence describing acute effects of sepsis is juxtaposed with the paucity of long-term data.
An animal study by Kaynar, et al. described an acceleration of atheroma formation in the aorta in five-month survivors of cecal ligation and puncture [38]. Confounders of the study included the involvement of only male animals deficient in apolipoprotein E [38]. Kaynar, et al. findings were consistent with the emergence of post-sepsis inflammatory syndrome, and is well established proof of post-sepsis atherosclerosis acceleration [1,6,8].
Van Leuvan, et al. demonstrated the suppression of HDL-c and LDL-c at four weeks after onset of sepsis in human survivors [39]. The decrease in HDL was accompanied by a modification in HDL composition with increased concertation of serum amyloid A during recovery [39]. This alteration in HDL-c composition is linked to increased platelet levels and monocyte activation potentially accelerating atherosclerosis [11,40]. In an independent investigation, Tanaka, et al. found a post-sepsis decrease in LDL-c, HDL-c and cholesterol 28 days post admission [41]. Similar data are seen in COVID-19 [42]. In all presented studies, the effect of sepsis on the acceleration of long-term lipid profiles was not the primary hypothesis. The scant data repetitively corroborate independently that even at 30 days post-sepsis, there are persistent, qualitative, and quantitative changes in the lipoprotein profile in survivors. However, long longitudinal studies are missing.
Surgery
The long-term effect of surgery on the lipid profile and natural evolution of atherosclerosis remains poorly described and almost impossible to distinguish from the effect of acute illness. In general, post-operative hypocholesteremia may persist over one year after surgery with unclear modification to the atherosclerosis progression [39,43]. In emergent surgery, cholesterol levels were noted to decline immediately [22,44]. The exception to this included neurological patients, where the level of HDL-c remains elevated, most likely secondary to the effect of phenytoin on HDL-c [45].
The clinical importance of these findings is obscure. Larsen, et al. suggested that accelerated atherosclerosis may be a reason for the increased prevalence of late cerebrovascular accidents after non-cardiac, non-carotid surgeries, however, most of the strokes occurred in the immediate post-operative period between days 5 and 26 [43]. It's important to note that this study was published in 1988 and the incidence of stroke was low (n = 6/2463). The increased incidence of embolic strokes was attributed to endothelial dysfunction rather than accelerated atherosclerosis [46].
Finally, we would like to note that bariatric surgery has been associated with a positive effect on the lipoprotein profile signified by an increase in HDL-c. This effect was inconsistent across studies and was believed to be due to the heterogeneity of the surgical technique used [47-49]. This net effect is most likely secondary to a change in dietary intake, not the persistent re-programming of the lipoprotein profile.
Trauma and splenectomy
Considering the fact that traumatic injuries have a higher prevalence within younger populations, abnormal changes within the lipid profile has the potential to have a more significant impact on long-term atherosclerosis. An acute decrease in lipoproteins after a traumatic injury is almost uniformly observed and linked to the degree of organ dysfunction [27].
There is a significant void in research involving long-term lipid profiles after a traumatic injury. We were able to locate three articles describing changes in the lipoprotein profile after a splenectomy. In most cases, splenectomy was the direct result of a traumatic injury, offering a natural, observational experiment in a very controlled way [50]. In general, data from human and animal studies demonstrated less atherosclerotic burden in the splenectomy patients. The long-term effects of a splenectomy induced the emergence of a pro-atherogenic lipoproteins that was at least partially diet related [51]. In particular, autopsies from 18 patients with a medical history spanning 20 years after a splenectomy demonstrate decreased macrophage density across all components of coronary arteries [52].
Concomitantly, an observational study of 740 United States service members subjected to splenectomies showed an excess in cardiovascular event incidence [53]. These results may suggest post-trauma changes in monocytes, may amplify rather lipoproteins abnormalities, as critical components of the atherosclerotic process [50]. The spleen is a critical partner in the modification of LDL particles and in monocyte turnover and activation [12,32,34,54].
ACS & Coronary Bypass Surgery
Despite numerous studies demonstrating the pivotal role of lipoprotein profile abnormalities in the development of atherosclerosis and the increased risk of acute coronary syndrome (ACS), little is known regarding how ACS as CCI affects the progress of atherosclerosis [9,10]. Acute changes in lipoproteins do not follow a "typical" pattern of change considering the fact that serum HDL-lipoprotein levels were not depressed during acute illness [55-57]. LDL-c was lower during acute ACS, but it is unclear how long these changes persisted [55-57]. This lipid profile predicted mortality acutely, but these findings are inconsistent with other studies demonstrating depression of LDL-c and HDL-c during acute phase of CCI [58].
We explored the effect of coronary artery bypass grafting (CABG) on the lipoprotein profile considering its correlation with ACS. In one study, 21 patients undergoing cardiac surgery with the application of cardiopulmonary bypass demonstrated a decrease in circulating LDL-c two days after insult, while HDL-c levels were somewhat less altered [59]. CABG was found to be related to a change in size of lipoproteins and a change in lipoprotein particle concentration. Other lipoprotein profile changes post-CABG included a decrease in total cholesterol levels by 35%, a decrease in triglycerides by 34.9%, and alteration in LDL as well as apoB [60]. These studies were the only two identified after the extensive literature search. Both studies focused on short-term outcomes. Despite the lack of extensive exploration into lipoprotein changes post-CABG, they may offer a clue regarding physiological changes similar to CCI post-CABG. The effect of cardiac surgery on lipoprotein levels will be methodologically challenging. Most of these patients have a rich medical history including statin therapy [61]. They have myriad of pre-existing conditions interfering with atherosclerotic process. However, the importance of assessing CABG's long-term impact on lipogram may be critical in order to advocate for less invasive procedures, like percutaneous angioplasty. Interestingly, less invasive procedure demonstrated similar outcomes to CABG in case of intervention driven by cardiac atherosclerosis in the first place.
Stroke and traumatic brain injury
The contribution of cholesterol abnormalities to the emergence of acute stroke is well established. Similar to ACS, we do not know if stroke itself results in the acceleration of atherosclerosis per se. Patients who suffer a stroke demonstrate several abnormalities distinctive from those typically observed in CCI [29-31]. In a prospective study involving 110 participants with cerebrovascular event, blood samples were collected at five different time-points: On admission, 24 hours post-event, 48 hours post-event, 72 hours post-event, and at discharge [62]. Participants were noted to have an initial 23% decrease in HDL-c, LDL-c and total cholesterol levels. By hour 24 post-event, the lipoprotein levels increased, but then quickly began to decline by hour 48 until discharge. The authors also found that lower HDL levels at 48 hours were associated with higher incidences of 90-day mortality rates. Lower HDL levels at discharge were associated with higher amounts of disability. Lastly, the study demonstrated that patients with larger amounts of stroke volume had a higher serum level of LDL and total cholesterol as compared to other CCI. Varela, et al. examined lipid profiles in patients three months after cerebrovascular accidents (CVA). They determined a diminished anti-inflammatory potency of HDL-c [63]. These dynamics of HDL-c in the wake of the cerebrovascular event were like those observed in other CCI. However, increase in LDL-c is a unique observation for CVA patients, not noted in other study involving CCI patients.
Traumatic brain injury (TBI) resulted in changes in lipoprotein profile even when it presents as a sole insult. Animal data demonstrated an increase in atherosclerosis burden and macrophage retention within the plaque after TBI [64]. A different study recruited a total of 553 veterans who were followed over four years and noted an increase in plaque formation, which led to an increased risk of atherosclerotic events [65]. The study did not investigate the lipoprotein profile specifically while this and other studies suggested a myriad of factors affecting excessive atherosclerosis [10,15,37,66,67]. Indirect evidence linking both TBI and abnormal lipoproteins profile was demonstrated by the relationship between lipoproteins profile and cytokine profile resulted in excessive 28 days mortality in TBI victims [68]. However, this study did not venture for more than one month of observation and it is correlational in its nature.
Acute liver failure
The liver is critical for lipid metabolism and is frequently affected in CCI. Trieb, et al. (2016) demonstrated changes in lipoprotein profile among patients with acutely decompensated cirrhosis. Significantly decreased cholesterol levels were observed, including LDL-c, HDL-c, and apoA-I, and increased levels of C-reactive protein [69]. Even patients with relatively preserved liver function were noted to have diminished HDL-c and apoA-I levels. They linked depletion of apo-B to a decline in HDL-c and ultimately mortality on a sample of 59 patients. Even after the resolution of the liver injury, the lipoprotein profile continues to demonstrate abnormalities for a prolonged period of time. In a prospective study that involved a total of 93 patients who underwent hepatectomy due to liver malignancies or liver cirrhosis, acute reduction of cholesterol on a postoperative day one and three were observed [70]. The majority of the patients survived the surgery without complication and were noted to have a subsequent rebound of cholesterol on day seven. In non-survivors, cholesterol levels continued to decline until death. Again, this is consistent with prior observations [30,44,55].
Conclusion
In summary, this review demonstrated that almost any CCI induces short-term lipoprotein abnormalities [20,22,33,57,68,69,71]. This is consistent with a high prevalence of metabolic abnormalities at the cellular level and the role of the lipoprotein in response to acute infections. Decline in HDL-c into recovery was the commonest mark across multitude of CCI. Some investigators pointed to an increase in serum VLDL-c, LDL-c, and elevated oxidation of circulating lipoprotein molecules. If these changes persist, the natural evolution of atherosclerosis may be accelerated [1,5,8]. Theoretically, the almost uniform decline in HDL suggests the emergence of an unfavorable lipoprotein profile by itself, but it is often accompanied by a concomitant decline in LDL, the risk of accelerated atherosclerosis is even higher. LDL-hypolipoproteinemia may lower the risk of atherosclerosis but decline in HDL may tamper this deleterious effect. At the same time, an increased rate of lipoprotein modification may offset any theoretical benefit of lower LDL-c [34]. Lack of consistent studies hampers any definite conclusions.
The overall impression is that post-CCI lipid milieu results most likely in a durable change in lipid profile extending to months after resolution of the acute illness process. No data for more than 6 months post-CCI lipid profile is present for any disease, which is one of the starkest findings of this manuscript. The data from animal studies strongly suggest long-term, deleterious effects on the lipogram and ultimately atherosclerosis in the aftermath of sepsis. Additional data from a patient suffering from long-term consequences of TBI and military staff after splenectomy further strengthen the case for the persistence of long-term lipid abnormalities [38,65,67]. However, the conclusion must be taken very carefully as the data from humans are somewhat opaque, showing variable to deleterious effects, and are correlational at best [20,29,51,57,59,60,65,66].
The persistence of unfavorable lipid profiles in patients after CCI suggests the therapeutic window to correct them in order to improve long-term outcomes. There are many agents to influence lipid profile, yet the question of whether prolonged post-septic abnormalities in the lipid profile can be effectively corrected with pharmacotherapy is not answered. Metabolic re-programming and the emergence of other pro-atherosclerotic conditions may hamper pharmacological interventions' benefit at reversing post-sepsis damage [72]. Due to the lack of adequate studies, this remains unknown.
One has to underline the critical difficulties in studying the presented topics. First, longitudinal studies are notoriously difficult to conduct. Second, the lipid profile is affected by diet and medications. The use of lipid-modifying drugs is common after stroke and ACS [61]. Third, the effects of CCI will be confounded by numerous variables, including the compliance with the therapy among the patients. Finally, atherosclerosis is a multifactorial disease. Besides lipid profiles, monocyte composition, monocyte activity, and overall endothelial function are critical and may independently alter the progression of atherosclerosis. All the reasons listed above stressed that we were forced to change the scope of our analysis. Our review was intended to be a systematic analysis from the onset of CCI. However, we quickly realized that there is not enough data to pursue our original methodology. Existing data is scarce and almost episodic for several patient populations with various endpoints, creating very high heterogeneity. This lack of well-grounded studies was surprising considering the increased interest in the health of the survivors. Furthermore, the advancements in medicine resulted in increased survivorship among the individuals coping with CCI. Considering the increased prevalence of survivorship and increased public interest, the lack of studies represents a significant knowledge gap that must be addressed. Lack of data limited the overall conclusion of this review to generalizations of few studies, indicating a knowledge gap that needs to be closed.
In summary, while few aspects of lipoprotein profile abnormalities have been studied in various critical care situations, there are significant gaps in knowledge evaluating the persistence and clinical importance of the long-term post-CCI lipid abnormalities. Additionally, future studies should assess the effects of lipoprotein profiles in the context of two main components of atherosclerosis progression: Monocytes and endothelium [1,8,10,73]. The current research suggests that CCI may have long-term effects on the lipoprotein profile; however, more definitive studies are needed considering the growing population of CCI survivors.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Funding
This research funded by NIH NIGMS K23 120630.
Authors' contribution
KS: Manuscript preparation, proofing; JH: Literature review, manuscript preparation, proofing; CR: Literature research; DL: Literature research; JM: Keyword creation, concept, manuscript; MR: Manuscript preparation; UP: Literature search; KL: Concept, literature search, original manuscript, proofing.
Authors' statement
All authors reviewed the final version of the manuscript and agreed for its publication.
Acknowledgments
Not applicable.
References
- Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nature reviews Disease primers 2016;2:16045.
- Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Brownstein BH, Mason PH, Baker HV, Finnerty CC, Jeschke MG, Lopez MC, Klein MB, Gamelli RL, Gibran NS, Arnoldo B, Xu W, Zhang Y, Calvano SE, McDonald-Smith GP, Schoenfeld DA, Storey JD, Cobb JP, Warren HS, Moldawer LL, Herndon DN, Lowry SF, Maier RV, Davis RW, Tompkins RG. A genomic storm in critically injured humans. J Exp Med 2011;208:2581-90.
- Hinson H, Schreiber M, Laurie A, Bourdette D. Early Fever Is Associated with Inflammation after TBI. Journal of Neurotrauma 2016;33:A21-A.
- Nowill AE, Fornazin MC, Spago MC, Dorgan Neto V, Pinheiro VRP, Alexandre SSS, Moraes EO, Souza G, Eberlin MN, Marques LA, Meurer EC, Franchi GC, Jr., de Campos-Lima PO. Immune Response Resetting in Ongoing Sepsis. J Immunol 2019;203:1298-312.
- Rajicic N, Cuschieri J, Finkelstein DM, Miller-Graziano CL, Hayden D, Moldawer LL, Moore E, O'Keefe G, Pelik K, Warren HS, Schoenfeld DA. Identification and interpretation of longitudinal gene expression changes in trauma. PLoS One 2010;5:e14380.
- Hotchkiss RS, Moldawer LL. Parallels between cancer and infectious disease. N Engl J Med 2014;371:380-3.
- Schuler A, Wulf DA, Lu Y, Iwashyna TJ, Escobar GJ, Shah NH, Liu VX. The Impact of Acute Organ Dysfunction on Long-Term Survival in Sepsis. Crit Care Med 2018;46:843-9.
- Mira JC, Brakenridge SC, Moldawer LL, Moore FA. Persistent Inflammation, Immunosuppression and Catabolism Syndrome. Crit Care Clin 2017;33:245-58.
- Witztum JL, Lichtman AH. The Influence of Innate and Adaptive Immune Responses on Atherosclerosis. Annual Review of Pathology: Mechanisms of Disease 2014;9:73-102.
- Goldberg IJ, Sharma G, Fisher EA. Atherosclerosis: Making a U Turn. Annual review of medicine 2020;71:191-201.
- Barlage S, Gnewuch C, Liebisch G, Wolf Z, Audebert FX, Glück T, Fröhlich D, Krämer BK, Rothe G, Schmitz G. Changes in HDL-associated apolipoproteins relate to mortality in human sepsis and correlate to monocyte and platelet activation. Intensive care medicine 2009;35:1877-85.
- Pirillo A, Norata GD, Catapano AL. LOX-1, OxLDL, and atherosclerosis. Mediators of inflammation 2013;2013:152786.
- Soran H, Durrington PN. Susceptibility of LDL and its subfractions to glycation. Current opinion in lipidology 2011;22:254-61.
- Hossain E, Ota A, Karnan S, Takahashi M, Mannan SB, Konishi H, Hosokawa Y. Lipopolysaccharide augments the uptake of oxidized LDL by up-regulating lectin-like oxidized LDL receptor-1 in macrophages. Mol Cell Biochem 2015;400:29-40.
- Dolmatova EV, Wang K, Mandavilli R, Griendling KK. The effects of sepsis on endothelium and clinical implications. Cardiovasc Res 2021;117:60-73.
- Forceville X, Van Antwerpen P, Preiser JC. Selenocompounds and sepsis: redox bypass hypothesis for early diagnosis and treatment Part A: Early acute phase of sepsis: an extraordinary redox situation (leukocyte-endothelium interaction leading to endothelial damage). Antioxid Redox Signal 2021.
- Kaysen GA, Ye X, Raimann JG, Wang Y, Topping A, Usvyat LA, Stuard S, Canaud B, van der Sande FM, Kooman JP, Kotanko P, Initiative obotMDO. Lipid levels are inversely associated with infectious and all-cause mortality: international MONDO study results. Journal of lipid research 2018;59:1519-28.
- Iribarren C, Jacobs D C, Sidney S, C.J. C, Feingold RC. Cohort study of serum total cholesterol and in-hospital incidence of infectious disease. Epidemiol Infect 1998;121:335-47.
- Feng Q, Wei W-Q, Chaugai S, Leon BGC, Mosley JD, Leon DAC, Jiang L, Ihegword A, Shaffer CM, Linton MF, Chung CP, Stein CM. Association Between Low-Density Lipoprotein Cholesterol Levels and Risk for Sepsis Among Patients Admitted to the Hospital With Infection. JAMA Network Open 2019;2:e187223-e.
- Chien Y-F, Chen C-Y, Hsu C-L, Chen K-Y, Yu C-J. Decreased serum level of lipoprotein cholesterol is a poor prognostic factor for patients with severe community-acquired pneumonia that required intensive care unit admission. Journal of Critical Care 2015;30:506-10.
- Pirillo A, Catapano AL, Norata GD. HDL in infectious diseases and sepsis. Handb Exp Pharmacol 2015;224:483-508.
- Lee SH, Lee JY, Hong TH, Kim BO, Lee YJ, Lee JG. Severe persistent hypocholesterolemia after emergency gastrointestinal surgery predicts in-hospital mortality in critically ill patients with diffuse peritonitis. PLoS One 2018;13:e0200187.
- Stevenson EK, Rubenstein AR, Radin GT, Wiener RS, Walkey AJ. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis*. Crit Care Med 2014;42:625-31.
- Stortz JA, Mira JC, Raymond SL, Loftus TJ, Ozrazgat-Baslanti T, Wang Z, Ghita GL, Leeuwenburgh C, Segal MS, Bihorac A, Brumback BA, Mohr AM, Efron PA, Moldawer LL, Moore FA, Brakenridge SC. Benchmarking clinical outcomes and the immunocatabolic phenotype of chronic critical illness after sepsis in surgical intensive care unit patients. J Trauma Acute Care Surg 2018;84:342-9.
- Stortz JA, Murphy TJ, Raymond SL, Mira JC, Ungaro R, Dirain ML, Nacionales DC, Loftus TJ, Wang Z, Ozrazgat-Baslanti T, Ghita GL, Brumback BA, Mohr AM, Bihorac A, Efron PA, Moldawer LL, Moore FA, Brakenridge SC. Evidence for Persistent Immune Suppression in Patients Who Develop Chronic Critical Illness After Sepsis. Shock 2018;49:249-58.
- Mangiola A, Vigo V, Anile C, De Bonis P, Marziali G, Lofrese G. Role and Importance of IGF-1 in Traumatic Brain Injuries. Biomed Res Int 2015;2015:736104.
- Dunham CM, Fealk MH, Sever WE, 3rd. Following severe injury, hypocholesterolemia improves with convalescence but persists with organ failure or onset of infection. Critical care (London, England) 2003;7:R145-53.
- Kumar V. Targeting macrophage immunometabolism: Dawn in the darkness of sepsis. Int Immunopharmacol 2018;58:173-85.
- Szeto CC, Kwan BC, Chow KM, Lai KB, Chung KY, Leung CB, Li PK. Endotoxemia is related to systemic inflammation and atherosclerosis in peritoneal dialysis patients. Clin J Am Soc Nephrol 2008;3:431-6.
- Lüthold S, Berneis K, Bady P, Müller B. Effects of infectious disease on plasma lipids and their diagnostic signi?cance in critical illnes. Europen Journal of Clinical Investigation 2007;37:573-9.
- Fraunberger P, Nagel D, Walli AK, Seidel D. Serum cholesterol and mortality in patients with multiple organ failure. Crit Care Med 2000;28:3574-5.
- Al-Banna N, Lehmann C. Oxidized LDL and LOX-1 in experimental sepsis. Mediators of inflammation 2013;2013:761789.
- Nishida M, Moriyama T, Ishii K, Takashima S, Yoshizaki K, Sugita Y, Yamauchi-Takihara K. Effects of IL-6, adiponectin, CRP and metabolic syndrome on subclinical atherosclerosis. Clin Chim Acta 2007;384:99-104.
- Lara-Guzman OJ, Gil-Izquierdo A, Medina S, Osorio E, Alvarez-Quintero R, Zuluaga N, Oger C, Galano JM, Durand T, Munoz-Durango K. Oxidized LDL triggers changes in oxidative stress and inflammatory biomarkers in human macrophages. Redox Biol 2018;15:1-11.
- Tanaka S, Diallo D, Delbosc S, Genève C, Zappella N, Yong-Sang J, Patche J, Harrois A, Hamada S, Denamur E, Montravers P, Duranteau J, Meilhac O. High-density lipoprotein (HDL) particle size and concentration changes in septic shock patients. Ann Intensive Care 2019;9:68.
- Behnes M, Brueckmann M, Liebe V, Liebetrau C, Lang S, Putensen C, Borggrefe M, Hoffmann U. Levels of oxidized low-density lipoproteins are increased in patients with severe sepsis. J Crit Care 2008;23:537-41.
- Bartolomé N, Aspichueta P, Martínez MJ, Vázquez-Chantada M, Martínez-Chantar ML, Ochoa B, Chico Y. Biphasic adaptative responses in VLDL metabolism and lipoprotein homeostasis during Gram-negative endotoxemia. Innate Immun 2012;18:89-99.
- Kaynar AM, Yende S, Zhu L, Frederick DR, Chambers R, Burton CL, Carter M, Stolz DB, Agostini B, Gregory AD, Nagarajan S, Shapiro SD, Angus DC. Effects of intra-abdominal sepsis on atherosclerosis in mice. Critical care (London, England) 2014;18:469.
- van Leeuwen HJ, Heezius EC, Dallinga GM, van Strijp JA, Verhoef J, van Kessel KP. Lipoprotein metabolism in patients with severe sepsis. Crit Care Med 2003;31:1359-66.
- Barlage S, Fröhlich D, Böttcher A, Jauhiainen M, Müller HP, Noetzel F, Rothe G, Schütt C, Linke RP, Lackner KJ, Ehnholm C, Schmitz G. ApoE-containing high density lipoproteins and phospholipid transfer protein activity increase in patients with a systemic inflammatory response. Journal of lipid research 2001;42:281-90.
- Tanaka S, Stern J, Bouzid D, Robert T, Dehoux M, Snauwaert A, Zappella N, Cournot M, Lortat-Jacob B, Augustin P, Atchade E, Tran-Dinh A, Meilhac O, Montravers P. Relationship between lipoprotein concentrations and short-term and 1-year mortality in intensive care unit septic patients: results from the HIGHSEPS study. Ann Intensive Care 2021;11:11.
- Tanaka S, De Tymowski C, Assadi M, Zappella N, Jean-Baptiste S, Robert T, Peoc'h K, Lortat-Jacob B, Fontaine L, Bouzid D, Tran-Dinh A, Tashk P, Meilhac O, Montravers P. Lipoprotein concentrations over time in the intensive care unit COVID-19 patients: Results from the ApoCOVID study. PLoS One 2020;15:e0239573.
- Larsen SF, Zaric D, Boysen G. Postoperative cerebrovascular accidents in general surgery. Acta Anaesthesiol Scand 1988;32:698-701.
- Gordon BR, Parker TS, Levine DM, Saal SD, Wang JC, Sloan BJ, Barie PS, Rubin AL. Relationship of hypolipidemia to cytokine concentrations and outcomes in critically ill surgical patients. Crit Care Med 2001;29:1563-8.
- Goerdt C, Keith M, Rubins HB. Effects of phenytoin on plasma high-density lipoprotein cholesterol levels in men with low levels of high-density lipoprotein cholesterol. Journal of clinical pharmacology 1995;35:767-75.
- Ng JLW, Chan MTV, Gelb Adrian W, Warner David S. Perioperative Stroke in Noncardiac, Nonneurosurgical Surgery. Anesthesiology 2011;115:879-90.
- Milone M, Lupoli R, Maietta P, Di Minno A, Bianco P, Ambrosino P, Coretti G, Milone F, Di Minno MN, Musella M. Lipid profile changes in patients undergoing bariatric surgery: a comparative study between sleeve gastrectomy and mini-gastric bypass. Int J Surg 2015;14:28-32.
- Puzziferri N, Roshek TB, 3rd, Mayo HG, Gallagher R, Belle SH, Livingston EH. Long-term follow-up after bariatric surgery: a systematic review. Jama 2014;312:934-42.
- Genua I, Ramos A, Caimari F, Balague C, Sanchez-Quesada JL, Perez A, Minambres I. Effects of Bariatric Surgery on HDL Cholesterol. Obesity surgery 2020.
- Ai XM, Ho LC, Han LL, Lu JJ, Yue X, Yang NY. The role of splenectomy in lipid metabolism and atherosclerosis (AS). Lipids Health Dis 2018;17:186.
- Akan AA, Sengül N, Simsek S, Demirer S. The effects of splenectomy and splenic autotransplantation on plasma lipid levels. J Invest Surg 2008;21:369-72.
- Li Y, Stone JR. The impact of splenectomy on human coronary artery atherosclerosis and vascular macrophage distribution. Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology 2016;25:453-60.
- Robinette CD, Fraumeni JF, Jr. Splenectomy and subsequent mortality in veterans of the 1939-45 war. Lancet 1977;2:127-9.
- Ferrante A, Drew PA, Kiroff GK, Zola H. Peripheral blood leucocyte subpopulations in patients splenectomized for trauma. Clin Exp Immunol 1987;70:158-63.
- Thakkar H, Vincent V, Roy A, Singh S, Ramakrishnan L, Kalaivani M, Singh A. HDL functions and their interaction in patients with ST elevation myocardial infarction: a case control study. Lipids Health Dis 2020;19:75.
- Kumar P, Singh S, Prasad s, Yadav UP, Agrawal PA. Study of lipid profile in acute myocardial infarction in 24 hours. Ann Appl Bio-Sciences 2018;5:A1-A8.
- Grion CM, Cardoso LT, Perazolo TF, Garcia AS, Barbosa DS, Morimoto HK, Matsuo T, Carrilho AJ. Lipoproteins and CETP levels as risk factors for severe sepsis in hospitalized patients. Eur J Clin Invest 2010;40:330-8.
- Górecki A, Bednarz B, Jaxa-Chamiec T, Maciejewski P, Lukaszewicz R, Ceremuzynski L, Dyduszynski A. Lipid profile during the first 24 hours after myocardial infarction has significant prognostic value. Kardiol Pol 2004;60:229-36; discussion 37.
- Hacquebard M, Ducart A, Schmartz D, Malaisse WJ, Carpentier YA. Changes in plasma LDL and HDL composition in patients undergoing cardiac surgery. Lipids 2007;42:1143-53.
- Hacquebard M, Ducart A, Schmartz D, Tembo N, Carpentier YA. Tocopherol in lipoproteins and blood cells after cardiac surgery. Annals of the New York Academy of Sciences 2004;1031:432-4.
- Hlatky MA, Solomon MD, Shilane D, Leong TK, Brindis R, Go AS. Use of medications for secondary prevention after coronary bypass surgery compared with percutaneous coronary intervention. Journal of the American College of Cardiology 2013;61:295-301.
- Perovic E, Mrdjen A, Harapin M, Simundic AM. Short Term Changes of Serum Lipids in Acute Ischemic Stroke. Clin Lab 2016;62:2107-13.
- Varela LM, Meseguer E, Lapergue B, Couret D, Amarenco P, Meilhac O. Changes in High-Density Lipoproteins Related to Outcomes in Patients with Acute Stroke. J Clin Med 2020;9.
- Wang J, Su E, Wang H, Guo C, Lawrence DA, Eitzman DT. Traumatic Brain Injury Leads to Accelerated Atherosclerosis in Apolipoprotein E Deficient Mice. Sci Rep 2018;8:5639.
- Ahmadi N, Hajsadeghi F, Yehuda R, Anderson N, Garfield D, Ludmer C, Vaidya N. Traumatic brain injury, coronary atherosclerosis and cardiovascular mortality. Brain Inj 2015;29:1635-41.
- Lekkou A, Mouzaki A, Siagris D, Ravani I, Gogos CA. Serum lipid profile, cytokine production, and clinical outcome in patients with severe sepsis. J Crit Care 2014;29:723-7.
- Johnson V, Stewart J, Smith D, Stewart W. Persistent Inflammation in the Corpus Callosum for Many Years Following a Single TBI in Humans. Journal of Neurotrauma 2012;29:A3-A.
- Venetsanou K, Vlachos K, Moles A, Fragakis G, Fildissis G, Baltopoulos G. Hypolipoproteinemia and hyperinflammatory cytokines in serum of severe and moderate traumatic brain injury (TBI) patients. Eur Cytokine Netw 2007;18:206-9.
- Trieb M, Horvath A, Birner-Gruenberger R, Spindelboeck W, Stadlbauer V, Taschler U, Curcic S, Stauber RE, Holzer M, Pasterk L, Heinemann A, Marsche G. Liver disease alters high-density lipoprotein composition, metabolism and function. Biochim Biophys Acta 2016;1861:630-8.
- Giovannini I, Chiarla C, Greco F, Boldrini G, Nuzzo G. Characterization of biochemical and clinical correlates of hypocholesterolemia after hepatectomy. Clin Chem 2003;49:317-9.
- Cirstea M, Walley KR, Russell JA, Brunham LR, Genga KR, Boyd JH. Decreased high-density lipoprotein cholesterol level is an early prognostic marker for organ dysfunction and death in patients with suspected sepsis. J Crit Care 2017;38:289-94.
- Gragnano F, Calabrò P. Role of dual lipid-lowering therapy in coronary atherosclerosis regression: Evidence from recent studies. Atherosclerosis 2018;269:219-28.
- Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science (New York, NY 2012;335:936-41.