Translational Perioperative and Pain Medicine (ISSN: 2330-4871)
ARTICLE DOI: 10.31480/2330-4871/126
Review Article OPEN ACCESS
The lateral Habenula: Role in Chronic Pain and Depression
Guang-Fen Zhang1,2, Jie Guo3, Jian-Jun Yang1*
1Department of Anesthesiology, Pain and Perioperative Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
2Department of Anesthesiology, Zhongda Hospital, School of Medicine, Southeast University, Nanjing, Jiangsu, China
3Department of Anesthesiology, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, Jiangsu, China
Jian-Jun Yang, M.D., Ph.D., Department of Anesthesiology, Pain and Perioperative Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China; E-mail: yjyangjj@126.com
Editor: Renyu Liu, MD, PhD, Professor, Department of Anesthesiology and Critical Care, Perelman School of Medicine at the University of Pennsylvania, Center of Penn Global Health Scholar, Director of Stroke 120 Special Task Force, Chinese Stroke Association, 336 John Morgan Building, 3620 Hamilton Walk, Philadelphia, PA 19104, USA, Phone: 2157461485, Fax: 2153495078, E-mail: RenYu.Liu@pennmedicine.upenn.edu
Received: May 05, 2020 | Accepted: June 29, 2020 | Published: July 07, 2020
Citation: Zhang G, Guo J, Yang J. The lateral Habenula: Role in Chronic Pain and Depression. Transl Perioper & Pain Med 2020; 7(4):271-278
Abstract
Pain and depression are highly prevalent and debilitating disorders that seriously affect certain individuals' quality of life. Clinical observations have long recognized the co-occurrence and interaction of pain and depression, and these disorders have been estimated to coexist in up to two-thirds of cases. However, the mechanisms underlying the comorbidity of pain and depression remain uncharacterized. The habenula is a conserved structure in the posterior-medial aspect of the dorsal thalamus that plays a crucial role in modulating midbrain monoaminergic systems. This structure receives input from forebrain structures, and its efferents primarily project into the midbrain and hindbrain, reaching well-known pain-modulating regions such as the periaqueductal gray matter and raphe nuclei as well as depression-modulating regions such as the dopaminergic ventral tegmental area and serotonergic dorsal raphe nucleus. Recently, numerous studies have indicated that the lateral habenula (LHb) is an important pain-processing region where external stimuli are received, evaluated, and redirected to motivate behavioral responses to pain. Furthermore, altered activity of the LHb is associated with depressive symptoms such as behavioral despair and anhedonia. Thus, increased activity of the LHb might play a key role in pain-depression comorbidity. Here, we review the interaction between pain and depression, describe the role of the habenula in each, and present evidence that the LHb is involved in the comorbidity of pain and depression.
Keywords
Lateral Habenula; Comorbidity; Chronic pain; Depression
Introduction
Chronic pain is defined as any pain lasting more than 3 months. In addition to physical reactions, chronic pain is often accompanied by negative emotions such as depression and anxiety, which seriously affect patients' quality of life [1-4]. Depression is one of the most common psychiatric disorders and is characterized by low mood and lack of pleasure. Additionally, the symptoms of depression usually include physical symptoms such as pain, insomnia, and fatigue [5,6]. Studies have shown that 30% to 100% of patients with chronic pain have depression, and 51.8% to 59.1% of patients with depression also report pain symptoms [7-9]. The comorbidity of pain and depression has long been recognized in the clinic, and the two conditions together are more difficult to treat than either condition alone. Antidepressants have been used to treat chronic pain since the 1960s, and analgesic treatments can alleviate the manifestation of depression in patients with pain symptoms [10,11].
Pain and depression share common neuroanatomical underpinnings, and the brain area affected by depression is also involved in the perception and processing of pain. The brain areas affected by both pain and depression include the insular cortex, medial prefrontal cortex, anterior cingulate cortex, hippocampus, habenula, amygdala, and thalamus [12-14]. The habenula is an evolutionarily ancient and centrally located nucleus that participates in a series of important biological processes [15]. The neural circuit composed of the habenula, periaqueductal gray, ventral tegmental area (VTA), and dorsal raphe nucleus (DRN) regulates norepinephrine (NE), serotonin (5-HT), dopamine (DA), and other neurotransmitter systems that are closely related to pain and depression [16-18]. Recent studies have suggested that the activation of the habenula and the subsequent changes in neurotransmitter activity caused by chronic pain are an important explanation for the promotion of depression-like behavior, and the activation of the habenula might also be a key factor causing pain sensitivity under conditions of depression [19,20]. As a core brain region that transmits information from the forebrain limbic system to the midbrain monoaminergic and dopaminergic neurons, the habenula might be an important region in the pathogenesis of pain-depression comorbidity and might also serve as a therapeutic target. Thus, this article summarizes the structural and functional changes in the habenula in chronic pain and depression, both individually and together, and explores the mechanism of habenular involvement in pain-depression comorbidity.
Comorbidity of Chronic Pain and Depression
Pain and depression are two highly prevalent disorders with significant socioeconomic impact. Studies have shown that the prevalence of chronic pain in adults is approximately 10%-30% [21-23], and depression is one of the most common mental disorders in the world, with a morbidity rate of approximately 17% [24]. Patients with chronic pain often develop depression-like behaviors such as decreased personal coping ability, reduced interest, and insomnia. All types of depression, especially major depressive disorder (MDD), are often accompanied by unexplained pain symptoms [6]. Clinical studies have shown a significant correlation between the degree of pain and the severity of depression. People with depression have higher pain scores than those without depression, and an increase in the degree of pain increases the negative emotions and behaviors of these patients [25,26]. A cross-sectional survey showed that nearly half of patients with MDD had at least one chronic pain symptom, and this rate was four times higher than the rate in the normal population [27]. A retrospective analysis showed that the average prevalence of pain symptoms in patients with depressive disorder was 65% [28]. A total of 77% of American outpatients with depression meet the criteria for pain diagnosis [29]. Previous studies showed that approximately 34% of patients with neuropathic pain suffer from depression [30], and approximately 22-26% of patients with fibromyalgia show depressive symptoms [31]. Gormsen et al. found that, compared with a healthy control group, patients with fibromyalgia and neuropathic pain had significantly increased depression and anxiety scores [32]. Additionally, recent studies have shown that patients with higher postoperative persistent pain scores have higher anxiety and depression scores [33].
The comorbidity of pain and depression is also well reflected in animal models. Animal models of pain can induce depression-like behavior, which is characterized by prolonged immobility time during forced swimming and reduced sucrose preference. Suzuki et al. [34] found that mice subjected to spinal nerve ligation showed mechanical allodynia 2 days after surgery and increased immobility time on the forced swimming test 15 days after surgery. Depression-like behavior occurred 2 to 4 weeks after chronic constriction injury (CCI) of the sciatic nerve in rats [35,36]. Increased immobility time in the FST occurred 5 weeks after spared nerve injury in rats [37,38]. Kim et al. found that the mechanical and thermal pain thresholds of rats were significantly decreased 2 weeks after a complete Freund's adjuvant (CFA) injection, and this change was accompanied by a significant increase in immobility time [39]. Borges et al. found that rats showed significant depression-like behaviors 28 days after CFA injection [40]. Previous studies found that animal models of both acute and chronic depression showed hyperalgesia and allodynia [41-44]. In another study, an acute depression rat model subjected to forced swimming showed increased hyperalgesia compared to the control group on the formalin test [41]. Suarez-Roca et al. showed that continuous forced swimming can lead to a reduction in the thermal pain threshold [42]. In an animal model of chronic depression caused by social defeat stress, both the chemical and mechanical pain thresholds were significantly reduced [43,44].
Previous studies have suggested that multiple brain regions (the cortex, hippocampus, amygdala, thalamus, VTA, DRN, etc.) and multiple molecular mechanisms (neuroendocrine dysfunction, hypofunction of monoaminergic systems, decreased neuronal regeneration, increased neuroinflammatory response and synaptic transmission dysfunction) are involved in the occurrence of pain-depression comorbidity [45-48]; however, the mechanism of this comorbidity has not been clearly elucidated.
The Habenula and its Afferent and Efferent Connections
The habenula (Hb) is a small bilateral structure located on the dorsal side of the diencephalon and on both sides of the third ventricle. This structure is divided into medial (MHb) and lateral (LHb) components, which differ in their neurochemical characteristics and connectivity. The habenula is an important hub that connects the limbic forebrain and midbrain, and it participates in the regulation of various physiological activities, including pain, depression, sleep, rhythm, learning, cognition, and reward [49-53]. The Hb is evolutionarily conserved from lower animals to higher animals, including humans, which indicates the importance of its role. Previous studies have shown that Hb is involved in a number of behavioral functions as mentioned above [49-55]. However, studies into the function of the MHb are limited. In recent years, the LHb has attracted great attention due to its important role in the pathogenesis of depression and pain.
The LHb receives and integrates inputs from the forebrain limbic system, including the prefrontal cortex, septum, globus pallidus (GP), bed nucleus of the stria terminalis (BNST), lateral preoptic area (LPO), diagonal band of Broca's area (DBB), lateral hypothalamus (LH), and basilar nucleus. The LHb sends projections to the serotonergic DRN and median raphe nucleus (MRN), the dopaminergic VTA and substantia nigra pars compacta (SNc), the GABAergic rostromedial tegmental nucleus (RMTg), and the periaqueductal gray (PAG) [49-53]. Neurons of the LHb also project to DA neurons of the VTA and SNc and 5-HT neurons of the DRN through the RMTg, resulting in an indirect inhibitory effect on midbrain DA and 5-HT cell activity [56,57]. Some of the dopaminergic neurons in the VTA and some of the serotoninergic neurons in the DRN also project back to the LHb, forming a feedback loop. Figure 1 illustrates the afferent and efferent fiber projections of the LHb.
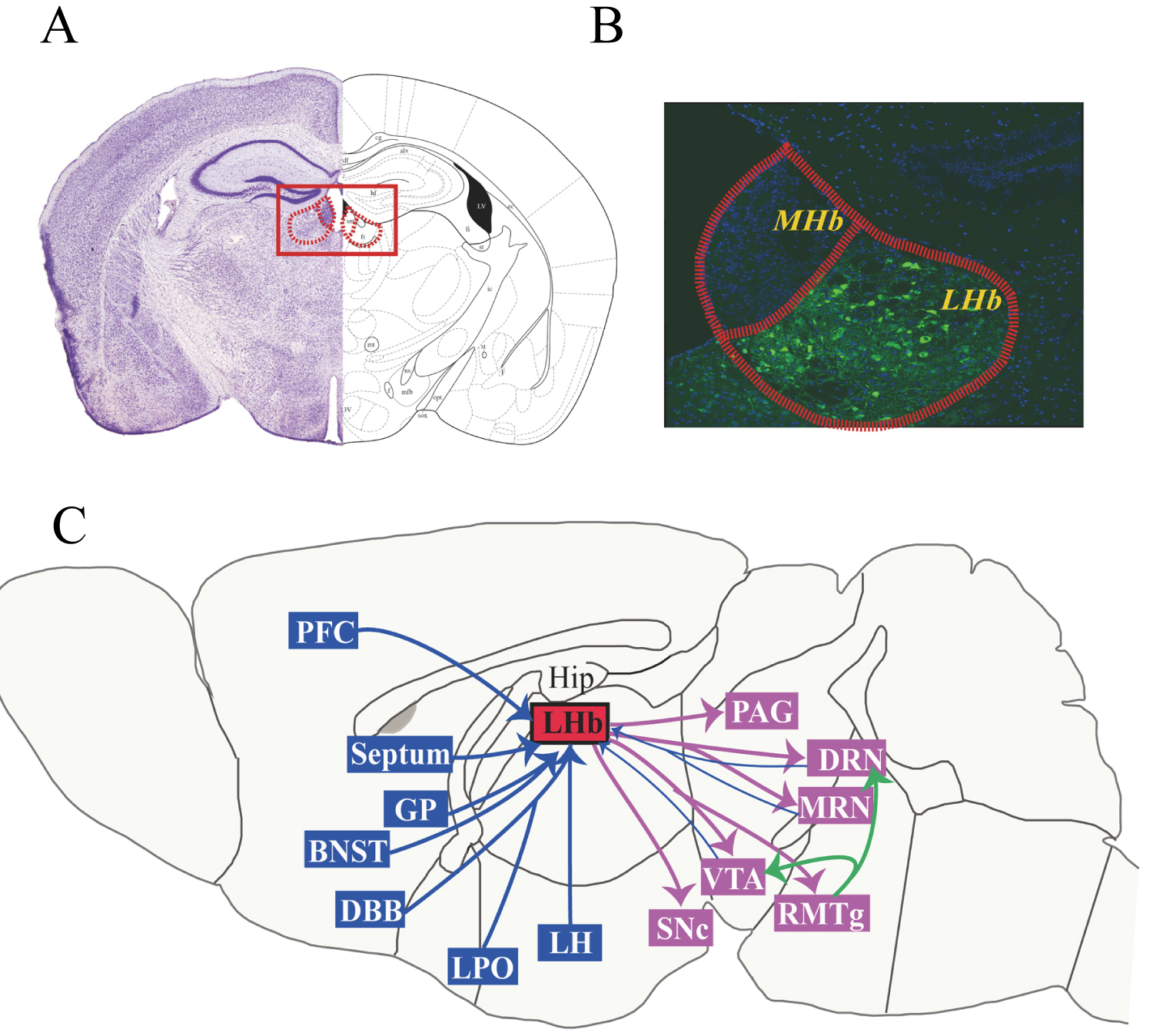
Figure 1: Afferent and efferent fiber projections of the LHb. Photomicrograph of a coronal section through a mid-rostrocaudal level of the rat habenula (A) and subnuclei of the MHb and LHb (B). Schematic diagram depicting major afferent and efferent connections of the LHb (C). Abbreviations: BNST, bed nucleus of the stria terminalis; DBB, diagonal band of Broca's area; DRN, dorsal raphe nucleus; GP, globus pallidus; Hip, hippocampus; LH, lateral hypothalamus; LHb, lateral habenula; LPO, lateral preoptic area; MHb, medial habenula; MRN, median raphe nucleus; NAc, nucleus accumbens; PAG, periaqueductal gray; PFC, prefrontal cortex; RMTg, rostro-medial tegmental nucleus; SNc, substantia nigra compacta; VTA, ventral tegmental area.
The Role of the Habenula in Pain
Shelton et al. conducted MRI studies of healthy volunteers and found that noxious thermal stimulation significantly enhanced habenula activity [58]. Studies based on resting-state fMRI suggest that the association between the habenula and the forebrain is significantly abnormal in children with complex regional pain syndrome. Basic research using animal models of chronic pain has shown a marked increase in blood flow to the habenula of rats [59,60]. Injecting morphine into the rat habenula relieves the acute inflammatory pain and trigeminal neuralgia caused by formalin [61-63]. Electrical stimulation of the habenula can be used to effectively treat the acute inflammatory pain caused by formalin [64]. After nociceptive stimulation, the expression of c-fos protein in the habenula increased significantly, which suggests that pain activates habenular neurons [19,65]. A previous result showed that the activity of the habenula was significantly enhanced after spinal cord injury using the 99mTc-hexamethylpropyleneamine oxime (99mTc-HMPAO) method to label neurons [66].
The habenula accepts both direct and indirect forms of ascending pain-transmitting fiber projection: direct transmission from lamina I of the spinal dorsal horn and the trigeminal nucleus and indirect transmission through the afferent fibers of the LH to the habenula. The habenula participates in the downward modulation of pain by regulating the functions of the PAG and DRN. The PAG is an important structure in the endogenous pain modulation system. Ma et al. showed that the habenular injection of naloxone blocked the analgesic effect of a PAG injection of morphine [67]. The DRN can participate in pain regulation by modulating the 5-HT system. A previous study has shown that the activation of the DRN region produces analgesic effects [68]. Hyperactivation of the LHb may inhibit the 5-HT neurons of the DRN through γ-aminobutyric acid (GABA) neurons in the RMTg to participate in pain.
The Role of the Habenular in Depression
The activation of the habenula is significantly increased in patients with depression and in various animal models of depression. Morris et al. [69] conducted PET imaging studies of patients with depression and found that when these patients developed depression symptoms, their habenula activity increased significantly. After deep brain stimulation (DBS) of the habenula to reduce the activity of the habenular neurons in clinical practice, the symptoms of some patients with treatment-resistant depression were greatly alleviated [70]. Caldecott-Hazard et al. [71] used 14C-labeled deoxyglucose to indicate the metabolic rates of different brain regions and found that the metabolism of the LHb was significantly increased in several animal models of depression. Shumake and Gonzalez-Lima [72] found that the cytochrome oxidase activity of the LHb was significantly upregulated in depressive animals. In 2013, Hu's research group found that the upregulation of βCaMKII in the LHb of animals with learned helplessness promotes the excessive excitability of the habenula by increasing the membrane GluR1, thereby enhancing the inhibition of the VTA and the DRN, which leads to the core symptoms of depression (e.g., anhedonia and behavioral despair) [18]. In that study, antidepressant therapy downregulated βCaMKII and improved depressive symptoms [18]. In 2018, this research group published two papers in the same issue of Nature. They found that under external stress stimulation, the expression of the Kir4.1 ion channel of a glial cell in the LHb was upregulated, decreasing the extracellular potassium ion concentration of neurons, promoting the conversion of neurons from a single to a clustered discharge pattern, and resulting in the excessive inhibition of downstream reward centers (including the VTA and DRN), thereby leading to depression [73,74]. The new antidepressant ketamine was able to exert a rapid antidepressant effect by blocking the bursting discharge of the LHb [73,74]. Li et al. [75] found that the state of learned helplessness enhances the discharge of LHb neurons in rats, and DBS selectively depletes glutamate from the LHb neurons projecting to the VTA dopaminergic neurons, significantly reducing learned helplessness. Injection of the GABAA receptor agonist muscimol in the LHb also ameliorates depression-like behavior in rats [76], and inhibiting the excitatory pathways of LHb neurons can be used to treat depression.
The habenula may participate in the pathological process of depression by enhancing the inhibition of DRN neurons and reducing 5-HT release. The basal level of 5-HT release by the raphe nucleus is low in depressed rats, and the metabolism and conversion rate of 5-HT can be improved by lesioning the LHb to alleviate depressive symptoms [77]. The above evidence indicates that the activity of the habenula is significantly increased in both patients with depression and animal models of depression; conversely, the inhibition of habenula activity can alleviate depressive symptoms.
The Role of the Habenula in Pain-Depression Comorbidity
Recent studies have found that the discharge activity of the habenula and the number of c-fos-positive cells increase in rat models (i.e., inflammatory pain + chronic unpredictable mild stress) of pain and depression comorbidity. Moreover, habenular damage can reduce pain-depression comorbidity [19]. In the CCI rat model, depressive symptoms occurred 28 days after hyperalgesia and were accompanied by an increase in habenular activity and a decrease in the 5-HT level in the DRN. Moreover, habenular damage may simultaneously improve the pain and depression caused by CCI [20]. Recently, Zhang's research group confirmed that SOMCeA neurons mainly innervate GluLHb neurons through glutamatergic projections using a viral tracing method, and their findings indicated that in mice with chronic pain and depressive-like symptoms, GluLHb neuronal activity is stimulated by increased SOMCeA glutamatergic inputs [78].
According to the pain-induced depression model, although hyperalgesia developed rapidly after tissue injury, depression-like manifestations usually appeared more slowly, following pain by 3 to 5 weeks [79]. It is unclear why depression takes this long to develop after hyperalgesia; however, the time-dependent relationship between hyperalgesia and depression is important for understanding the neuropathological mechanisms through which chronic pain causes depression.
Does a time-dependent change occur in the habenula and its downstream projection targets after tissue injury to mediate the time difference between the aforementioned symptoms? Studies of persistent noxious stimulation have shown that the LHb initially acts as a relay station for nociceptive transmission and modulation; when this region receives nociceptive information from the spinal cord, voltage-dependent calcium channels open, increasing the intracellular calcium ion concentration [2,19]. Calcium ions act as a second messenger to promote the synthesis and release of many neuropeptides closely related to pain. Under the condition of acute pain, activity increases in the habenula and all associated circuits, which is a homeostatic response to a reversible physiological stimulus. Under the condition of chronic pain, the continuously activated spinal cord-habenula connection disrupts the related nerve projections and the homeostasis of the circuits. Eventually, the neural circuits associated with the reward responses of the VTA and DRN are suppressed, leading to a decrease in the release of 5-HT and DA, which causes depressive symptoms to appear [2]. Figure 2 illustrates the role of the habenula in pain-induced depression. Likewise, the activity of the habenula is increased in depression, and it modulates pain by regulating the functions of the PAG and DRN, leading to hyperalgesia.
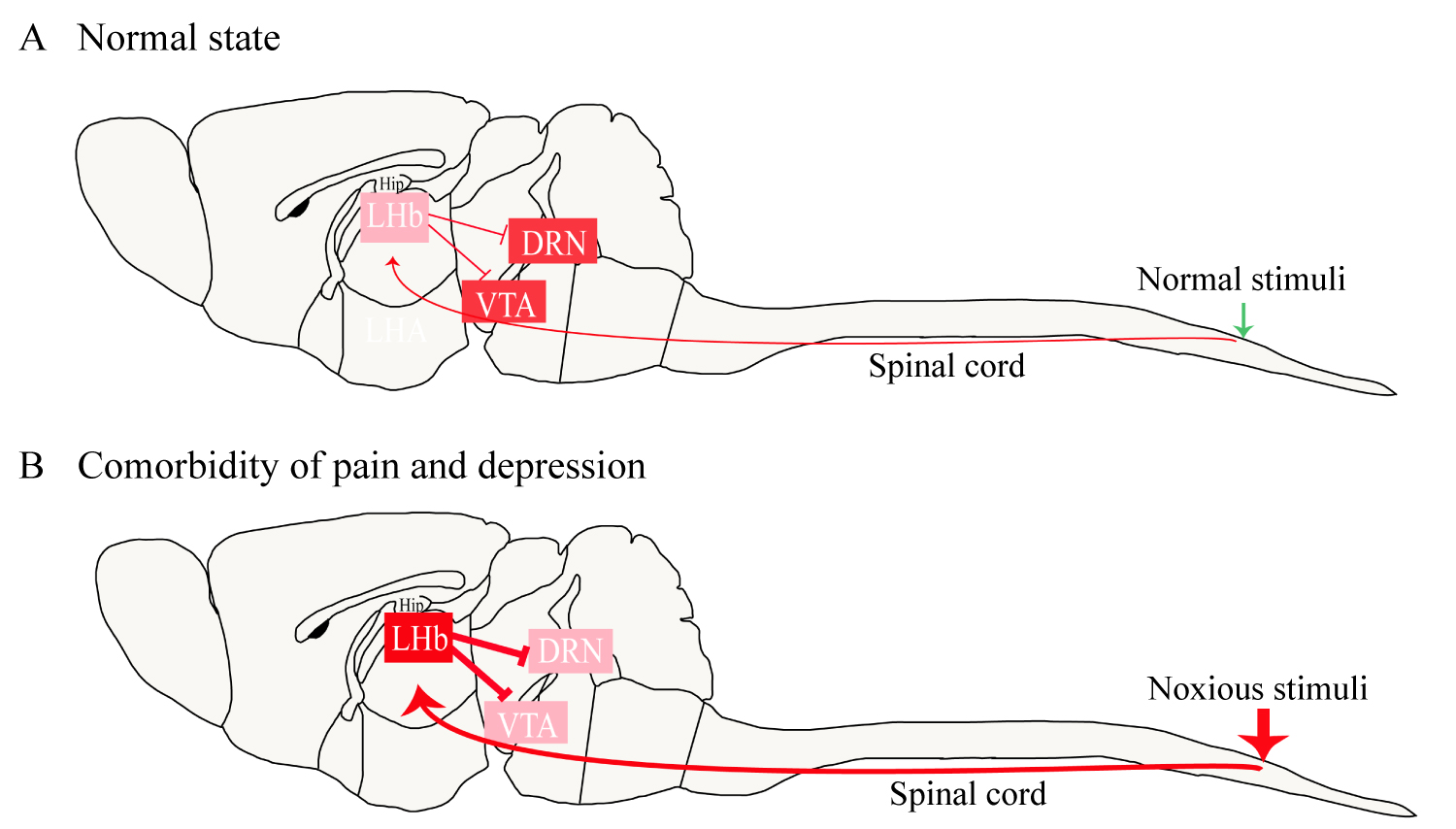
Figure 2: The role of the habenula in pain-induced depression. Under normal circumstances, the LHb provides a low level of inhibition to the VTA and DRN (A). In a state of pain-induced depression, noxious stimulation continues to activate the habenula, resulting in a significant increase in the excitability of the LHb neurons (B). The high excitability of the LHb enhances the inhibition of the VTA and DRN, leading to manifestations of depression such as anhedonia and behavioral despair.
The expression of the βCaMΚΙΙ is significantly up-regulated in the LHb of animal in depressed state and βCaMKII overexpression in the LHb can increase miniature excitatory postsynaptic currents in the LHb and induce depression-like behaviors in rats [18]. Li et al. demonstrates that βCaMKII expression in the LHb is increased in pain-associated depression rats model [20]. These results suggest that βCaMKII may be an important molecule involved pain-depression comorbidity. Some studies also indicates that the dopamine receptors, melatonin receptors, and p11 are strongly linked to LHb hyperactivation and maybe associated with pain-depression comorbidity [80-82].
Conclusions
Previous researches on chronic pain have primarily focused on the upstream nociceptive transmission pathways (e.g., the dorsal root ganglia and tractus spinothalamicus) and the downstream nociceptive regulation systems of spinal dorsal horn, the medulla oblongata, and midbrain. However, chronic pain is often accompanied by negative emotions such as depression; thus, the study of emotion-related brain regions has received increasing research attention in relation to the pain-depression comorbidity. Previous studies have suggested that the cortex, hippocampus, amygdala, thalamus, VTA, and DRN are involved in the pathogenesis of pain and depression. As an important relay station linking the forebrain and midbrain, the LHb receives projections from many brain regions involved in the pain-related stimuli, and sends outputs to well-known depression-modulating regions such as the dopaminergic VTA and serotonergic DRN. The important anatomical location of the habenula and its regulation of various neurotransmitter systems suggest that it is closely related to pain-depression comorbidity. LHb neurons are activated under conditions of both pain and depression, and these conditions can be relieved after inhibiting or destroying the habenula. The complex changes of LHb neural circuits may involve in pain-depression comorbidity. However, the cellular and molecular mechanisms linking the habenula to pain-depression comorbidity remain unclear, and researches in this area remain in the initial stage, calling for further exploration. Researches on the changes in the habenula for pain-depression comorbidity might provide new ideas with regard to the mechanisms as well as scientific treatment guidance for this comorbidityin clinical practice.
Funding Sources
The present study was supported by the National Natural Science Foundation of China (No. 81971020, 81771156, and 81503053).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
References
- Baliki MN, Apkarian AV. Nociception, pain, negative moods, and behavior selection. Neuron. 2015; 87(3):474-491.
- Shelton L, Becerra L, Borsook D. Unmasking the mysteries of the habenula in pain and analgesia. Prog Neurobiol. 2012; 96(2):208-219.
- Felice VD, Moloney RD, Cryan JF, Dinan TG, O'Mahony SM. Visceral pain and psychiatric disorders. Mod Trends Pharmacopsychiatry. 2015; 30:103-119.
- Zis P, Daskalaki A, Bountouni I, Sykioti P, Varrassi G, Paladini A. Depression and chronic pain in the elderly: links and management challenges. Clin Interv Aging. 2017; 12:709-720.
- Battle DE. Diagnostic and statistical manual of mental disorders: DSM-5. Codas. 2013; 25(2):191-192.
- Jaracz J, Gattner K, Jaracz K, Górna K. Unexplained painful physical symptoms in patients with major depressive disorder: prevalence, pathophysiology and management. CNS Drugs. 2016; 30(4):293-304.
- Miller LR, Cano A. Comorbid chronic pain and depression: Who is at risk? J Pain. 2009; 10:619-627.
- Agüera-Ortiz L, Failde I, Mico JA, Cervilla J, López-Ibor JJ. Pain as a symptom of depression: prevalence and clinical correlates in patients attending psychiatric clinics. Journal of Affective Disorders. 2011; 130:106-112.
- Lee P, Zhang M, Hong JP, Chua HC, Chen KP, Tang SW, et al. Frequency of painful physical symptoms with major depressive disorder in Asia: relationship with disease severity and quality of life. Journal of Clinical Psychiatry. 2009; 70:83-91.
- Młyniec K, Gaweł M, Doboszewska U, Starowicz G, Pytka K, Davies CL, et al. Essential elements in depression and anxiety. Part II. Pharmacol Rep. 2015; 67(2):187-194.
- Yalcin I, Barrot M. The anxiodepressive comorbidity in chronic pain. Curr Opin Anaesthesiol. 2014; 27(5):520-527.
- Wiech K, Tracey I. The influence of negative emotions on pain: behavioral effects and neural mechanisms. Neuroimage. 2009; 47(3):987-994.
- Mutschler I, Ball T, Wankerl J, Strigo IA. Pain and emotion in the insular cortex: evidence for functional reorganization in majordepression. Neurosci Lett. 2012; 520(2):204-209.
- Strobel C, Hunt S, Sullivan R, Sun J, Sah P. Emotional regulation of pain: the role of noradrenaline in the amygdala. Sci China Life Sci. 2014; 57(4):384-390.
- Bianco IH, Wilson SW. The habenular nuclei: a conserved asymmetric relay station in the vertebrate brain. Philos Trans R Soc Lond B Biol Sci. 2009; 364(1519):1005-1020.
- Elman I, Borsook D, Volkow ND. Pain and suicidality: insights from reward and addiction neuroscience. Prog Neurobiol. 2013; 109:1-27.
- Han S, Yang SH, Kim JY, Mo S, Yang E, Song KM, et al. Down-regulation of cholinergic signaling in the habenula induces anhedonia-like behavior. Sci Rep. 2017; 7(1):900.
- Li K, Zhou T, Liao L, Yang Z, Wong C, Henn F, et al. βCaMK in lateral habenula mediates core symptoms of depression. Science. 2013; 341 (6149): 1016 -1020.
- Li J, Li Y, Zhang B, Shen X, Zhao H. Why depression and pain often coexist and mutually reinforce: Role of the lateral habenula. Exp Neurol. 2016, 284(Pt A):106-113.
- Li Y, Wang Y, Xuan C, Li Y, Piao L, Li J, et al. Role of the lateral habenula in pain-associated depression. Front Behav Neurosci. 2017; 21;11:31.
- Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006; 10(4):287-333.
- Boulanger A, Clark AJ, Squire P, Cui E, Horbay GL. Chronic pain in Canada: have we improved our management of chronic noncancer pain? Pain Res Manag, 2007; 12(1): 39-47.
- Institute of medicine. Committee on advancing pain research C, education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. National Academies Press, 2011.
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003; 289:3095-3105.
- Jaracz J, Gattner K, Jaracz K, Górna K. Unexplained painful physical symptoms in patients with major depressive disorder: prevalence, pathophysiology and management. CNS Drugs. 2016; 30(4):293-304.
- Hiyama A, Watanabe M, Katoh H, Sato M, Sakai D, Mochida J. Effect of depression and neuropathic pain using questionnaires on quality of life in patients with low back pain; cross-sectional retrospective study. Eur Spine J. 2016; 25(9):2750-2760.
- Ohayon MM, Schatzberg AF. Using chronic pain to predict depressive morbidity in the general population. Arch Gen Psychiatry. 2003; 60:39-47.
- Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003; 163:2433-2445.
- Husain MM, Rush AJ, Trivedi MH, McClintock SM, Wisniewski SR, Davis L, et al. Pain in depression: STAR*D study findings. J Psychosom Res. 2007; 63:113-122.
- Gustorff B, Dorner T, Likar R, Grisold W, Lawrence K, Schwarz F. Prevalence of self-reported neuropathic pain and impact on quality of life: a prospective representative survey. Acta Anaesthesiol Scand. 2008; 52: 132-136.
- Epstein SA, Kay G, Clauw D, Heaton R, Klein D, Krupp L, et al. Psychiatric disorders in patients with fibromyalgia. A multicenter investigation. Psychosomatics. 1999; 40:57-63.
- Gormsen L, Rosenberg R, Bach FW, Jensen TS. Depression, anxiety, health-related quality of life and pain in patients with chronic fibromyalgia and neuropathic pain. Eur J Pain. 2010; 14(2):127.e1-8.
- Rockett M, Creanor S, Squire R, Barton A, Benger J, Cocking L, et al. The impact of emergency department patient-controlled analgesia (PCA) on the incidence of chronic pain following trauma and non-traumatic abdominal pain. Anaesthesia. 2019; 74(1):69-73.
- Suzuki T, Amata M, Sakaue G, Nishimura S, Inoue T, Shibata M. Mashimo TExperimental neuropathy in mice is associated with delayed behavioral changes related to anxiety and depression. Anesth Analg. 2007; 104(6):1570-1577.
- Fukuhara K, Ishikawa K, Yasuda S, Kishishita Y, Kim HK, Kakeda T, et al. Intracerebroventricular 4-methylcatechol (4-MC) ameliorates chronic pain associated with depression-like behavior via induction of brain-derived neurotrophic factor (BDNF). Cell Mol Neurobio. 2012; 32(6):971-977.
- Hu B, Doods H, Treede RD, Ceci A. Depression-like behaviour in rats with mononeuropathy is reduced by the CB2-selective agonist GW405833. Pain. 2009; 143(3):206-212.
- Jesse CR, Wilhelm EA, Nogueira CW. Depression-like behavior and mechanical allodynia are reduced by bis selenide treatment in mice with chronic constriction injury: a comparison with fluoxetine, amitriptyline, and bupropion. Psychopharmacology (Berl). 2010; 212(4):513-522.
- Leite-Almeida H, Pinto-Ribeiro F, Almeida A. Animal Models for the Study of Comorbid Pain and Psychiatric Disorders. Mod Trends Pharmacopsychiatry. 2015;30:1-21.
- Kim H, Chen L, Lim G, Sung B, Wang S, McCabe MF, et al. Brain indoleamine 2,3-dioxygenase contributes to the comorbidity of pain and depression. J Clin Invest. 2012; 122(8):2940-2954
- Borges G, Neto F, Mico JA, Berrocoso E. Reversal of monoarthritis-induced affective disorders by diclofenac in rats. Anesthesiology. 2014; 120(6):1476-1490.
- Quintero L, Cardenas R, Suarez-Roca H. Stress-induced hyperalgesia is associated with a reduced and delayed GABA inhibitory control that enhances post-synaptic NMDA receptor activation in the spinal cord. Pain. 2011; 152(8):1909-1922.
- Suarez-Roca H, Silva JA, Arcaya JL, Quintero L, Maixner W, Pinerua-Shuhaibar L. Role of mu-opioid and NMDA receptors in the development and maintenance of repeated swim stress-induced thermal hyperalgesia. Behav Brain Res. 2006; 167(2):205-211.
- Rivat C, Becker C, Blugeot A, Zeau B, Mauborgne A, Pohl M. Chronic stress induces transient spinal neuroinflammation, triggering sensory hypersensitivity and long-lasting anxiety-induced hyperalgesia. Pain. 2010; 150(2):358-368.
- Pagliusi MOF Jr, Bonet IJM, Dias EV, Vieira AS, Tambeli CH, Parada CA, et al. Social defeat stress induces hyperalgesia and increases truncated BDNF isoforms in the nucleus accumbens regardless of the depressive-like behavior induction in mice. Eur J Neurosci. 2018 Jun 9. [Epub ahead of print]
- Maletic V, Raison CL. Neurobiology of depression, fibromyalgia and neuropathic pain. Front Biosci. 2009; 14:5291-5338.
- Yalcin, Barthas F, Barrot M. Emotional consequences of neuropathic pain: Insight from preclinical studies. Neurosci Biobehav Rev. 2014; 47: 154-164.
- Alba-Delgado C, Llorca-Torralba M, Horrillo I, Ortega JE, Mico JA, Sánchez-Blázquez P, et al. Chronic pain leads to concomitant noradrenergic impairment and mood disorders. Biol Psychiatry. 2013; 73(1): 54-62.
- Norman GJ, Karelina K, Zhang N, Walton JC, Morris JS, Devries AC. Stress and IL-1beta contribute to the development of depressive-like behavior following peripheral nerve injury. Mol Psychiatry. 2010; 15(4): 404-414.
- Herkenham M, Nauta WJ. Afferent connections of the habenular nuclei in the rat. A horseradish peroxidase study, with a note on the fiber-of-passage problem. J Comp Neurol. 1977; 173(1): 123-146.
- Herkenham M, Nauta WJ. Efferent connections of the habenular nuclei in the rat. J Comp Neurol. 1979; 187(1): 19-47.
- Kowski AB, Geisler S, Krauss M, Veh RW. Differential projections from subfields in the lateral preoptic area to the lateral habenular complex of the rat. J Comp Neurol. 2008; 507(4): 1465-1478.
- Shabel SJ, Proulx CD, Trias A, Murphy RT, Malinow R. Input to the lateral habenula from the basal ganglia is excitatory, aversive, and suppressed by serotonin. Neuron. 2012; 74(3): 475-481.
- Hikosaka O. The habenula: from stress evasion to valuebased decision-making. Nat Rev Neurosci. 2010; 11(7): 503-513.
- Andres KH, von Düring M, Veh RW. Subnuclear organization of the rat habenular complexes. J Comp Neurol. 1999;407(1):130-50.
- Baker PM, Mizumori SJY. Control of behavioral flexibility by the lateral habenula. Pharmacol Biochem Behav. 2017;162:62-68.
- Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. J Comp Neuro. 2009; 513(6): 597-621.
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009; 61(5): 786-800.
- Shelton L, Pendse G, Maleki N, Moulton EA, Lebel A, Becerra L, et al. Mapping pain activation and connectivity of the human habenula. J Neurophysiol. 2012; 107: 2633-2648.
- Paulson PE, Morrow TJ, Casey KL. Bilateral behavioral and regional cerebralblood flow changes during painful peripheral mononeuropathy in the rat. Pain. 2000; 84:233-245.
- Paulson PE, Casey KL, Morrow TJ. Long-term changes in behavior and regionalcerebral blood flow associated with painful peripheral mononeuropathy inthe rat. Pain. 2002; 95:31-40.
- Cohen SR, Melzack R. Morphine injected into the habenula and dorsal posteromedial thalamus produces analgesia in the formalin test. Brain Res. 1985; 359(1-2): 131-139.
- Cohen SR, Melzack R. Habenular stimulation produces analgesia in the formalin test. Neurosci let. 1986; 70(1): 165-169.
- Khalilzadeh E, Saiah GV. The possible mechanisms of analgesia produced by microinjection of morphine into the lateral habenula in the acute model of trigeminal pain in rats. Res Pharm Sci. 2017; 12(3): 241-248.
- Mahieux G, Benabid AL. Naloxone-reversible analgesia induced by electrical stimulation of the habenula in the rat. Brain Res. 1987; 406(1-2): 118-129.
- Nagao M, Kamo H, Akiguchi I, Kimura J. Induction of c-Fos-like protein in the lateral habenular nucleus by persistent noxious peripheral stimulation. Neurosci Lett. 1993 Mar 5;151(1):37-40.
- Paulson PE, Wiley JW. Morrow TJ. Concurrent activation of the somatosensory forebrain and deactivation of periaqueductal gray associated with diabetes induced neuropathic pain. Exp Neurol. 2007; 208(2): 305-313.
- Ma QP, Shi YS, Han JS. Further studies on interactions between periaqueductal gray, nucleus accumbens and habenula in antinociception. Brain Res. 1992; 583:292-295.
- Mayer DJ. Analgesia produced by electrical stimulation of the brain. Prog Neuropsychopharmacol Biol Psychiatry. 1984; 8:557-564.
- Morris JS, Smith KA, Cowen PJ, Friston KJ, Dolan RJ. Covariation of activity in habenula and dorsal raphe nuclei following tryptophan depletion. Neuroimage. 1999; 10(2): 163-172.
- Sartorius A, Kiening KL, Kirsch P, von Gall CC, Haberkorn U, Unterberg AW, et al. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010; 67(2): e9-11.
- Caldecott-Hazard S, Mazziotta J, Phelps M. Cerebral correlates of depressed behavior in rats, visualized using 14C-2-deoxyglucose autoradiography. J Neurosci. 1988; 8(6): 1951-1961.
- Shumake J, Gonzalez-Lima F. Brain systems underlying susceptibility to helplessness and depression. Behav Cogn Neurosci Rev. 2003; 2(3): 198-221.
- Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S, et al. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature. 2018; 554(7692):317-322.
- Cui Y, Yang Y, Ni Z, Dong Y, Cai G, Foncelle A, et al. Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature. 2018; 554(7692):323-327.
- Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, et al. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011; 470(7335): 535 -539.
- Winter C, Vollmayr B, Djodari-Irani A, Klein J, Sartorius A. Pharmacological inhibition of the lateral habenula improves depressive-like behavior in an animal model of treatment resistant depression. Behav Brain Res. 2011; 216(1): 463 -465.
- Yang LM, Hu B, Xia YH, Zhang BL, Zhao H. Lateral habenula lesions improve the behavioral response in depressed rats via increasing the serotonin level in dorsal raphe nucleus. Behav Brain Res. 2008; 188(1):84-90.
- Zhou W, Jin Y, Meng Q, Zhu X, Bai T, Tian Y, et al. A neural circuit for comorbid depressive symptoms in chronic pain. Nat Neurosci. 2019; 22(10):1649-1658.
- Yalcin I, Bohren Y, Waltisperger E, Sage-Ciocca D, Yin JC, Freund-Mercier MJ, et al. A time-dependent history of mood disorders in a murine model of neuropathic pain. Biol Psychiatry. 2011; 70(10): 946-953.
- Seo JS, Zhong P, Liu A, Yan Z, Greengard P. Elevation of p11 in lateral habenula mediates depression-like behavior. Mol Psychiatry. 2018;23(5):1113-1119.
- Chan J, Ni Y, Zhang P, Zhang J, Chen Y. D1-like dopamine receptor dysfunction in the lateral habenula nucleus increased anxiety-like behavior in rat. Neuroscience. 2017;340:542-550.
- Evely KM, Hudson RL, Dubocovich ML, Haj-Dahmane S. Melatonin receptor activation increases glutamatergic synaptic transmission in the rat medial lateral habenula. Synapse. 2016;70(5):181-186.
Table of Contents
- Abstract
- Keywords
- Introduction
- Comorbidity of Chronic Pain and Depression
- The Habenula and its Afferent and Efferent Connections
- The Role of the Habenula in Pain
- The Role of the Habenular in Depression
- The Role of the Habenula in Pain-Depression Comorbidity
- Conclusions
- Funding Sources
- Conflict of Interest Statement
- Figure 1
- Figure 2
- References