Translational Perioperative and Pain Medicine (ISSN: 2330-4871)
ARTICLE DOI: 10.31480/2330-4871/113
Research Article OPEN ACCESS
Remifentanil Reduces Oxidative Stress and Inflammation and Attenuates Endothelial-Myocardial Injury in Patients Undergoing Mitral Valve Replacement Surgery
Chun CHEN1*, Xiaowen MAO2, Lei LIN1, Peng YANG1, Mingquan CHEN1, Jun HOU1, Shujun WANG1, Heqing TANG1, Han LIN3 and Zhengyuan XIA2,3
1Department of Anesthesiology, YiChang Central People's Hospital, The First College of Clinical Medical Science, Three Gorges University, Yichang, China
2Department of Anesthesiology, The University of Hong Kong, China
3Department of Anesthesiology, the Second Affiliated Hospital & Yuying Children's Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, China
Dr. Chun Chen, Department of Anesthesiology, YiChang Central People's Hospital, The First College of Clinical Medical Science, Three Gorges University, Yichang, China, E-mail: haohaoma-6@163.com
Editor: Renyu Liu, MD, PhD, Associate Professor, Department of Anesthesiology and Critical Care, Perelman School of Medicine at the University of Pennsylvania, Center of Penn Global Health Scholar, Director of Stroke 120 Special Task Force, Chinese Stroke Association, 336 John Morgan building, 3620 Hamilton Walk, Philadelphia, PA 19104, USA, Phone: 2157461485, Fax: 2153495078, Email: RenYu.Liu@pennmedicine.upenn.edu
Received: January 23, 2020 | Accepted: February 01, 2020| Published: February 10, 2020
Citation: CHEN C, MAO X, LIN L, YANG P, CHEN M. Remifentanil Reduces Oxidative Stress and Inflammation and Attenuates Endothelial-Myocardial Injury in Patients Undergoing Mitral Valve Replacement Surgery. Transl Perioper & Pain Med 2020; 7(2):200-207.
Abstract
Background
Circulating endothelial cells (CEC) are sensitive markers and direct evidence of endothelial damage induced by cardiopulmonary bypass (CPB). Remifentanil preconditioning (RP) has been demonstrated to attenuate CPB-induced organ damage, while whether the protection is related to reducing endothelial damage is unknown. This study aimed to investigate RP protection on vascular endothelial cells of patients undergoing open-heart surgery using CPB.
Methods
50 patients undergoing mitral valve replacement surgery were randomly assigned either to the RP group (n = 25) or the control group (C, n = 25). Perioperative data were recorded and blood samples were obtained preoperatively (baseline, pre-CPB, 5 minutes, 1, 6, 12 and 24 hours post aortic-cross-clamping). CEC-release and parameters of endothelial function (vWillebrand-Factor (vWF), soluble-thrombomodulin (sTM)), inflammation (Interleukin-6, TNFα), oxidative stress (MDA), myocardial cellular injury (CK-MB, troponin-I) and cerebral injury (S-100β) were measured.
Results
Levels of CEC-release, parameters of myocardial (troponin-I, CK-MB) and cerebral injury (S-100β) were significantly increased after reperfusion compared to baseline or pre-CPB that were concomitant with significant increases in interleukin-6, TNFα and MDA (all P < 0.05). RP significantly attenuated the increase of all the above mentioned parameters as early as 5 minutes or 1 hour after reperfusion (aortic declamping) and onwards and improved post-operative cardiac function evidenced as significant enhancement of cardiac index (all P < 0.05 vs. control group).
Conclusion
Remifentanil attenuates endothelial-myocardial injury in patients undergoing mitral valve replacement surgery and the mechanism might be attributable to reduction in oxidative stress and inflammation during myocardial ischemia and reperfusion.
Keywords
Remifentanil preconditioning, Cardiopulmonary bypass, Circulating endothelial cells, Endothelial injury
Introduction
With the development of new techniques such as cardiopulmonary bypass (CPB) to facilitate open-heart surgery the mortality rate due to myocardial infarctions, heart failure, or fatal arrhythmia after surgery has decreased significantly [1]. However, post-ischemic reperfusion injury occurred during CPB is a major cause of endothelial cell activation and injury [2-4] and may lead to multi-system organ dysfunction [5,6]. Vascular endothelial cell injury has been considered to be the initiating factor in cardiovascular disease [7,8]. Circulating endothelial cells (CEC) is endothelial cells detaching from the surface of tissue into the peripheral blood vessels and is a sensitive marker of endothelial damage with the value significantly elevated during CPB procedures. Thus, reducing CEC release is a key strategy in reducing CPB-induced endothelial cell damage and improving the long-term efficacy for patients during CPB.
Remifentanil preconditioning (RP), achieved by intravenous remifentanil infusion interspersed with infusion-free periods before ischemia, has been shown to attenuate post-ischemic reperfusion injury [9-13] in many organs. RP protection on CPB-induced myocardial [14] or cerebral [15] injury was also reported. However, very few study correlated RP effect on organ protection to the prevention of endothelial activation. Activation and injury of endothelial cells as a result of the contact of blood and artificial surfaces in the heart-lung machine during CPB has been considered as a key process in the manifestation of a whole body inflammatory reaction secondary to CPB [16] and oxidative stress initiated during myocardial ischemia-reperfusion injury [17].
In the present study, we aimed to evaluate the organ and endothelial protection of RP in patients undergoing open-heart surgery with CPB and explore its potential association with systemic inflammation and oxidative stress.
Material and Methods
Patients
This study protocol was approved by the Institutional Ethical Committee at YiChang Central People's Hospital, YiChang, China (Clinical trial registration number: ChiCTR-IPR-15005829) and informed written consent was obtained from all study subjects. This clinical trial was performed in a total of 50 patients with American Society of Anesthesiologists physician status II to III and the same director of cardiothoracic surgery physician surgeon completed all the surgery. Patients were recruited according to the following criteria: All patients were scheduled for mitral valve replacement performed via CPB. Exclusion criteria included patients with cancer, liver and kidney dysfunction; patients taking immunosuppressants, cytotoxic drugs, hormone replacement therapy and patients using aspirin, warfarin and heparin before surgery. Sample size was estimated based on differences in CEC levels measured at 1 hour post-ACC in a pilot study. Sample size was calculated by PASS 11 software, with α = 0.05 and power = 0.8, which determined that the study would be adequately powered with n = 22 per group. We subsequently decided to recruit a total of 50 patients to allow potential drop off. Patients were randomly allocated to either the remifentanil preconditioning (RP, n = 25) or control group (C, n = 25) by opening an envelope.
Anesthetic protocols and surgery
All patients received standard premedication of scopolamine at 0.006 mg/kg and morphine at 0.1 mg/kg intramuscularly 30 min before surgery. In all groups, anesthesia was induced with midazolam at 0.05 mg/kg, etomidate at 0.3 mg/kg, fentanyl at 10 μg/kg and vecuronium at 0.15 mg/kg given intravenously. After induction, all patients received continuous infusions of fentanyl at rate of 0.6 μg·kg−1·min−1 and vecuronium at 2 μg·kg−1·min−1 during surgery. During anesthesia, patients were monitored by GE solor8000 with five-lead ECG, pulse oximetry, capnography, invasive arterial pressure and pulmonary artery pressure during the operation.
Surgery was conducted under conditions of Mild hypothermia CPB (patient Rectal temperature of 28-32 °C) with STOCKERT-III type cardiopulmonary bypass machine, which uses Xijing membrane oxygenator with no blood prefilled. 4:1 oxygenated blood intermittent cold crystalloid cardioplegia perfusion was applied. All patients during CPB received heparin at dosage of 3 mg/kg, priming 10 mg/L, and activated clotting time (ACT) during CPB was maintained above 480s during CPB. MAP (mean arterial blood pressure) was maintained between 50 and 70 mmHg during CPB. Heparin was neutralized with protamine after separation from CPB at a ratio of 1:1.
Remifentanil (4 μg·kg−1·min−1) was continuously infused for 5 min, repeated for three cycles at 5 min interval before CPB in the RP groups; the same volume of saline was infused in C group.
Analysis of CEC frequency
CEC frequency in the peripheral blood was determined by MACS [18]. Briefly, arterial blood samples were collected in 2, 7 ml EDTA tubes, and stored at 4 °C for a maximum of 24 h for later batch analysis. The monoclonal mouse anti-human CD146 antibody (Biocytex, Marseilles, France) was conjugated to anti-mouse IgG beads (Dynal M-450, Norway) according to the manufacturer's instructions. After cleaning the magnetic beads, they were resuspended and mixed with an anti-CD146 monoclonal antibody, then diluted to a final concentration of 1.4 × 108 beads/ml using the PBS/BSA. 1 ml of whole blood was added into 20 ul cleaned antibody anti-CD146 beads, and samples were incubated on a rotator (10 rpm) for 30 min at 4 °C. An equal volume of buffer, placed in a magnetic rack for 2 min, and the remaining liquid was aspirated off the cells bound to magnetic beads, washing was repeated four times according to the instructions. Then, the cells were resuspended in 100 μl binding buffer and beads with acridine orange staining, fluorescence inverted phase contrast microscope counting. Another central venous 3.6 ml, 0.4 ml was added to plastic tubes containing sodium citrate in the mix, and samples were centrifuged on a rotator (3000 rpm) for 10 min at 4 °C, the upper plasma was drew to store at -20 °C. Criteria defining a CEC were: Fluorescein positive, 15-30 μm diameter of cell body and bound to at least 4 dynabeads [19]. The total number of CEC was normalized to a volume of one ml of peripheral blood.
Hemodynamic evaluation
All patients were monitored by invasive hemodynamic assessment using a pulmonary artery catheter. Data were recorded prior to CPB (pre-CPB), 5 min, 1 h, 6 h, 12 h and 24 h after aortic declamping.
Pumping dopamine or dobutamine (5 μg·kg−1·min−1) and milinone (0.375 μg·kg−1·min−1) before cardiac repulsion is our routine procedures for mitral valve replacement surgery.
Dopamine or dobutamine dosage was increased to treat a mean radial arterial blood pressure less than 65 mmHg despite optimization of preload, afterload and HR (heart rate). Using of dopamine or dobutamine (> 5 ug·kg−1·min−1) for a duration of 30 minutes or longer was recorded as the need for inotropic support.
Serological analyses
Serological evaluation of patients' blood was performed respectively prior to CPB (pre-CPB), 5 min, 1h, 6h, 12h and 24h after aortic declamping, which include cardiac enzymes creatine kinase -MB (CK-MB), tumor necrosis factor alpha (TNFα), troponin-I (cTnI) and S-100β protein. Additionally, von-Willebrand factor antigen (vWF) was measured by enzyme-linked immunosorbent assay using the vWF ELISA kit (Jiancheng Bioengineering Institute, Nanjing, China). For determination of soluble thrombomoduline concentration (sTM), a commercial solid phase sandwich enzyme-linked immunsorbent assay kit was used (sTM ELISA kit. Jiancheng Bioengineering Institute, Nanjing, China). S-100β protein was measured by enzyme-linked immunosorbent assay using the S-100β ELISA kit (Jiancheng Bioengineering Institute, Nanjing, China). Plasma malondialdehyde (MDA) and serum IL-6 were measured by commercial kits (Jiancheng Bioengineering Institute, Nanjiang, China).
Statistical analysis
All data were analyzed by SPSS13.0 statistical software. Non-normal data are expressed as the median and analyzed after log conversion data. Measurement data were expressed as mean ± S.D. Descriptive statistics were computed for variables of interest and analyzed using univariate ANOVA. Continuous data were analyzed using ANOVA with repeated measures. Significance was assumed with a p-value < 0.05.
Results
General data
Patients' perioperative demographics are summarized in Table 1 and Table 2. There was no statistical difference in patient characteristics in terms of age, gender, weight, aortic cross-clamp time, ventilation time, transfusion requirements and total intensive care stay between C and RP groups. Generally, no mortality, perioperative stroke was observed during the entire study period. Fewer patients in RP group required cardioversion to restore rhythm after CPB than in C group (P < 0.05). In addition, fewer patients in RP group required postoperative moderate inotropic support compared with the C group. Fewer overall chest tube drainage was showed in RP group (P < 0.05 vs. C). However, perioperative incidence of transitory psychotic disorder syndromes was low and equally distributed in both groups.
Table 1: Patient demographic data.
| Variable | Group C | Group RP | P value |
|
Gender (males/female) Age (years) Weight (kg)LVEF (%) Diabetes (n) Hypertension (n) COPD (n) Etiology of mitral diseases Severe mitral stenosis (n) Severe mitral regurgitation (n) Mixed valve disease (n) Preoperative medication Diuretics (n) β-Blockers (n) Digoxin (n) |
12/13 50 ± 12 56 ± 7 51 ± 9 2 1 2
5 3 17
20 9 12 |
11/14 47 ± 13 55 ± 10 54 ± 8 3 2 2
4 2 19
18 10 14 |
NS NS NS NS NS NS NS
NS NS NS
NS NS NS |
Table 2: Intra-operative and postoperative characteristics of patients.
| Variable | Group C | Group RP | P value |
|
Intra-operative data CPB time (min) Aortic cross-clamp time (min) Operative time (min) Rectal temperature (℃) Patients requiring cardioversion to restore rhythm after CPB (n) Postoperative data Dopamine or dobutamine > 5 ug·kg−1·min−1 (n) Transitory psychotic disorder syndrome Chest tube drainage (24h) Transfusion of Red Blood Cells (u) Transfusion of Fresh Frozen Plasma (ml) Duration of ventilation (h) Intensive care stay (h) Hospital stay (day) Mortality (n) Stroke (n) |
75 ± 12 62 ± 12 272 ± 31 29.6 ± 1.1 6
8 2 449 ± 131 1.0 ± 1.2 174 ± 152 3.2 ± 0.8 24.2 ± 8.4 12 ± 4 0 0 |
81 ± 14 61 ± 15 281 ± 29 29.2 ± 0.8 1
3 1 356 ± 98 0.8 ± 1.1 154 ± 130 2.9 ± 0.8 22.8 ± 9.6 1.1 ± 3 0 0 |
NS NS NS NS < 0.05
< 0.05 NS < 0.05 NS NS NS NS NS NS NS |
Hemodynamic evaluation
Patients' hemodynamics heart rate (HR), mean arterial blood pressure (MAP) and central venous pressure (CVP) over time did not significantly differ between groups. Cardiac index (CI) value was significantly higher at 6 h after reperfusion in RP group compared to baseline or pre-CPB values (P < 0.05). However, CI improvement in C group occurred 6 h later than RP group. CI value was significantly higher in RP group than that in C group (P < 0.05) during early reperfusion (1h and 6 h post-ACC) (Figure 1).
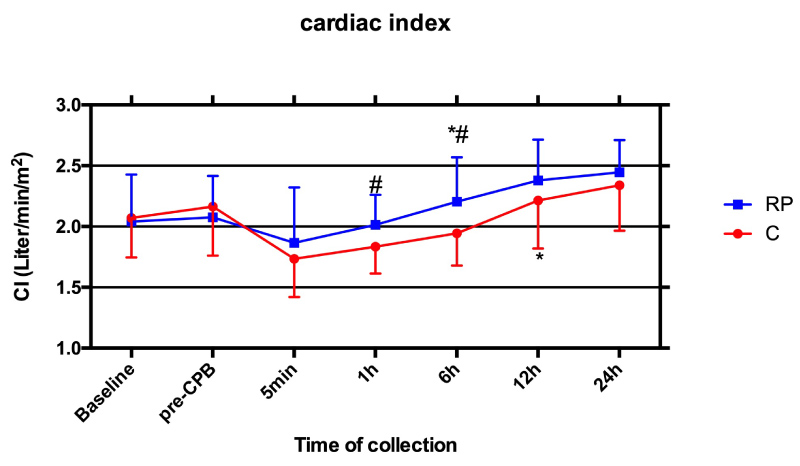
Figure 1: Perioperative cardiac index (CI): Serial time course of CI measured by pulmonary artery catheter in Control group (C, red line) and Remifentanil preconditioning group (RP, blue line). Values are means ± S.D. *P < 0.05 or P < 0.01 compared with Baseline and pre-CPB; #P < 0.05 compared with C.
Circulating Endothelial Cells (CEC)
As showed in Figure 2, preoperative CEC numbers (cells per milliliter of blood) did not differ between two groups. After perfusion, CEC value was significantly elevated in both RP and C groups compared to baseline or pre-CPB (P < 0.05), at 5 min, 1h, 6h and 12h post-ACC and peaked at 1h after perfusion. CEC numbers in RP group was significantly less than that in C group (P < 0.05) during early reperfusion (5 min, 1h and 6h post-ACC).
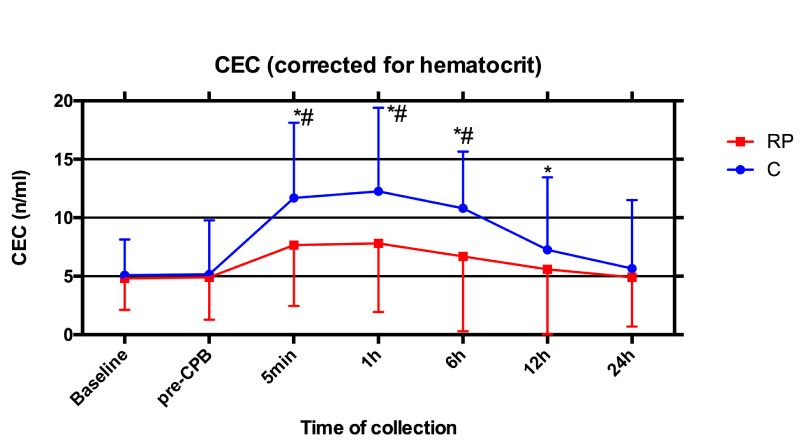
Figure 2: Perioperative numbers of circulating endothelial cells (CEC): Serial time course of CEC values in Control group (C, blue line) and Remifentanil preconditioning group (RP, red line). Values are means ± S.D. *P < 0.05 or P < 0.01 compared with Baseline and pre-CPB; #P < 0.05 compared with C.
Serology
As shown in Figure 3A-Figure 3C, TNFα, MDA and IL-6 concentrations didn't differ between group C and group RP at baseline and pre-CPB. MDA and IL-6 steady increased till 1h post-ACC while TNFα concentration started to decrease at 6h post-ACC (P < 0.05 compared with Baseline and pre-CPB). After reperfusion, all parameters in group RP were significantly lower than that in group C till they reached peak value (P < 0.05).
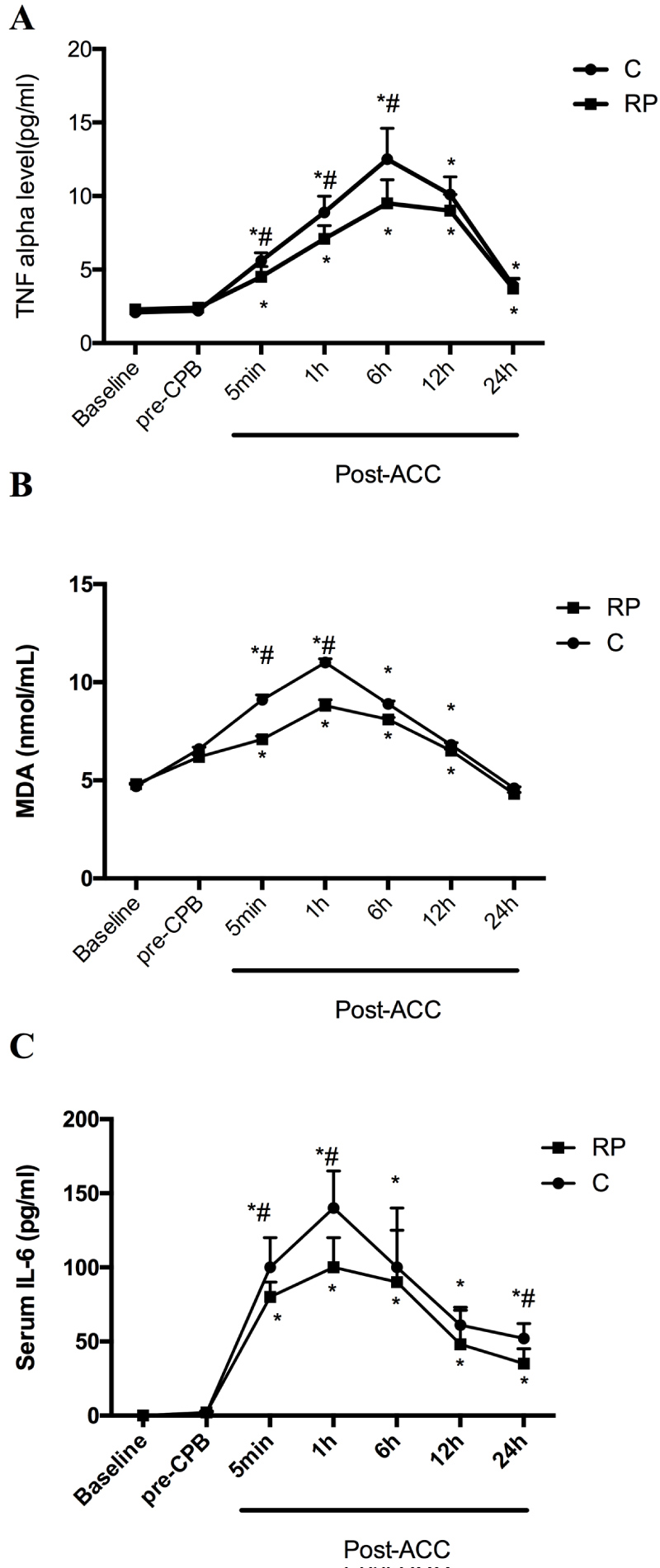
Figure 3: Perioperative serological evaluation of patients: Serial time course of TNFα (A), MDA (B) and IL-6 (C). (B), cTnI (C), S100β (D), vWF (E) and sTM (F) in Control group (C) and Remifentanil preconditioning group (RP). Values are means ± S.D. *P < 0.05 or P < 0.01 compared with Baseline and pre-CPB; #P < 0.05 compared with C.
Baseline and pre-CPB serum levels of CK-MB, cTnI, S-100β, vWF and sTM did not differ between groups, but concentrations of all the above parameters significantly increased upon reperfusion at 5 min post-ACC and onwards (P < 0.05 compared with Baseline and pre-CPB). Level of CK-MB peaked at 12h, S-100β peaked at 6h and cTnI, vWF and sTM peaked at 1h post-ACC, respectively in both RP and C groups. After reperfusion, plasma CK-MB, cTnI and S-100β concentrations in group RP were significantly lower than that in group C respectively (P < 0.05). Serum levels of CK-MB, S-100β and cTnI was significantly increased in both the RP group and C group starting from 5 min post-ACC while significant elevation of vWF and sTM levels were not apparent until after 1h post-ACC (All P < 0.05 vs. C, Figure 4) in both groups and the values of vWF and sTM in RP groups was significantly lower than that in C group.
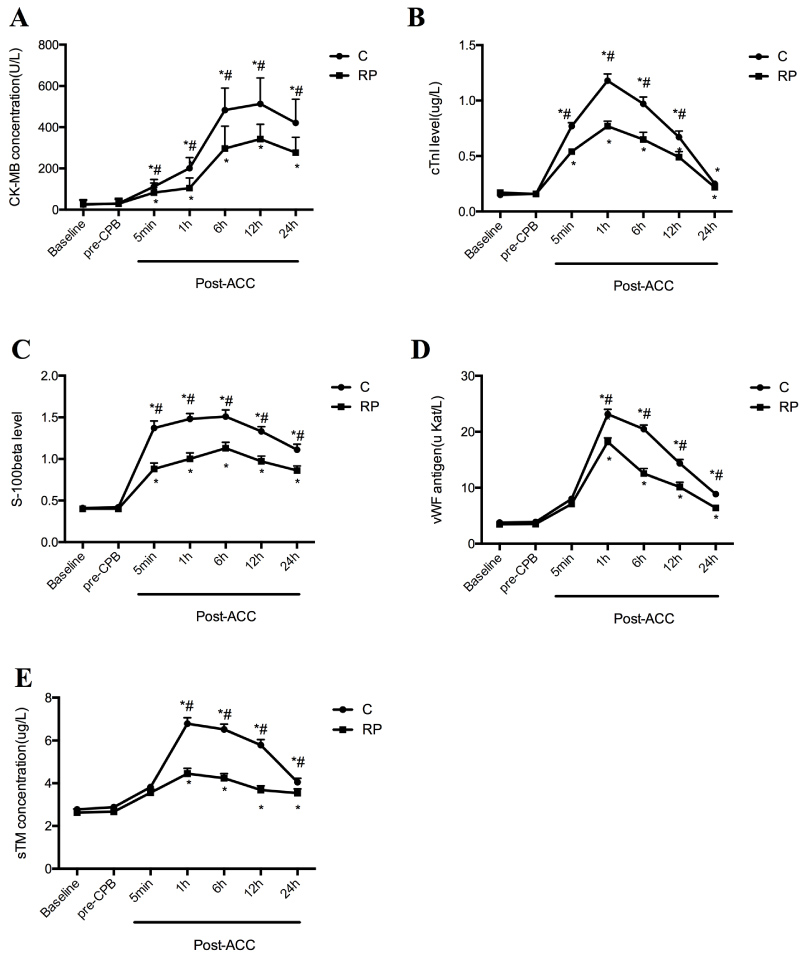
Figure 4: Perioperative serological evaluation of patients: Serial time course of CK-MB (A), cTnI (B), S100β (C), vWF (D) and sTM (E) in Control group (C) and Remifentanil preconditioning group (RP). Values are means ± S.D. *P < 0.05 or P < 0.01 compared with Baseline and pre-CPB; #P < 0.05 compared with C.
Discussion
In the present study, we showed that patients with CPB had a significantly more pronounced endothelial activation/injury demonstrated by the numbers of circulating endothelial cells. Also, serum derived from patients after CPB exerted an increased concentration of cardiac injury indicators (TNFα, MDA, CK-MB and cTnI), inflammatory cytokine IL-6 and brain injury indicator S100β compared to Baseline and pre-CPB period. And, the above elevations are tightly associated with the myocardial dysfunction as the cardiac index dropped during reperfusion. RP conferred significant organ protection evidenced as significantly attenuated the CPB-induced myocardial and brain injury as well as systemic inflammation and this protection may correlated with the effect on ameliorating endothelial activation/injury showed by less CEC release and lower vWF and sTM levels after RP treatment.
CEC may be the most direct marker of endothelial activation/injury [20]. Since these cells are very rarely found in the blood of healthy people, the increased number reflects the degree of endothelial activation or damage in patients after a period of CPB [21,22]. RP successfully attenuated the levels of CECs from early reperfusion (5 min post-ACC) and the effect was most profound at 1h and throughout the observational period of 24 h. The effect of RP on the reduction of CEC release should be attributable to the effects of RP in attenuating myocardial and cerebral injury. This is evidenced as the increase of post-operative CI and the decreases in TNFα, MDA, CK-MB, IL-6, cTnI and S100β after reperfusion in patients with treated with RP. Of note, the effect of RP on ameliorating endothelial injury is postponed to 1h post-ACC and this may explained as that CEC acted as an endothelial injury initiating factor and the complete endothelial restoration by RP has not yet been accomplished at the time when CEC release dropped.
Remifentanil-based anesthesia has been shown to have significant advantages for fast-track cardiac surgery [23] given the rapid clearance of remifentanil [24]. In addition, the metabolism of remifentanil is not affected by plasma cholinesterase and anti-cholinesterase drugs and the regression of drug action is mainly due to rapid clearance of the drug without redistribution [25]. Thus, it avoids accumulation problems during anesthesia with drugs like fentanyl, alfentanil, sufentanil, etc. after a long time of intravenous infusion. The myocardial and cerebral protections of remifentanil make RP a promising and a novel avenue for the prevention of organ damage in patients undergoing cardiac surgery with CPB.
The study should be considered worthy of further investigation in larger studies, because the relatively small sample size might represent a limitation to our conclusions. However, the major finding of the present investigation is the fact that RP can reduce the release of CEC, reducing endothelial injury, resulting in effects of organ protective and less morbidity in open-heart surgery. It should be noted that due to the differences in different valve replacement procedures, including surgical methods, CPB time, and other hemodynamic characteristics, we conducted this study purposely in patients undergoing mitral valve replacement surgery. We believe that theoretically the remifentanil cardioprotective effects could also be achieved in other open heart surgeries under cardiopulmonary bypass, but this needs to be further confirmed.
Conclusion
Remifentanil preconditioning can reduce the release of CEC, reducing endothelial injury, resulting in effects of organ protective and less morbidity in open-heart surgery.
Authors Contribution
Chen C performed experiment, analyzed data and wrote the paper; Mao XW analyzed data and wrote the paper; Lin L performed experiment and analyzed data; Yang P and Hou J performed experiment; Chen MQ analyzed data. Wang SJ and Lin H analyzed data; Xia Z analyzed the data and revised the manuscript.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Kirklin JK, Westaby S, Blackstone EH, Kirklin JW, Chenoweth DE, et al. Complement and the damaging effects of cardiopulmonary bypass. J Thorac Cardiovasc Surg 1983. 86(6): 845-57. PMID: 6606084
- Nagareddy PR, Xia Z, McNeill JH, MacLeod KM. Increased expression of iNOS is associated with endothelial dysfunction and impaired pressor responsiveness in streptozotocin-induced diabetes. Am J Physiol Heart Circ Physiol 2005. 289(5): H2144-52. PMID: 16006542
- Chen Q , Jin M, Yang F, Zhu J, Xiao Q, et al. Matrix metalloproteinases: inflammatory regulators of cell behaviors in vascular formation and remodeling. Mediators Inflamm 2013. 2013: 928315. PMID: 23840100
- Al-Fares A, Pettenuzzo T, Del Sorbo L. Extracorporeal life support and systemic inflammation. Intensive Care Med Exp 2019. 7(Suppl 1): 46. PMID: 31346840
- Wittwer T , Choi YH, Neef K, Schink M, Sabashnikov A, et al. Off-pump or minimized on-pump coronary surgery--initial experience with Circulating Endothelial Cells (CEC) as a supersensitive marker of tissue damage. J Cardiothorac Surg 2011. 6: 142. PMID: 22011515
- Xia Z, Chen Y, Fan Q, Xue M. Oxidative stress-mediated reperfusion injury: mechanism and therapies. Oxid Med Cell Longev 2014. 2014: 373081. PMID: 24803980
- Lee HZ, Yeh FT, Wu CH. The effect of elevated extracellular glucose on adherens junction proteins in cultured rat heart endothelial cells. Life Sci 2004. 74(17): 2085-96. PMID: 14969714
- Chen Z, Hu Z, Lu Z, Cai S, Gu X, et al. Differential microRNA profiling in a cellular hypoxia reoxygenation model upon posthypoxic propofol treatment reveals alterations in autophagy signaling network. Oxid Med Cell Longev 2013. 2013: 378484. PMID: 24454982
- Kim HS, Cho JE, Hong SW, Kim SO, Shim JK, et al. Remifentanil protects myocardium through activation of anti-apoptotic pathways of survival in ischemia-reperfused rat heart. Physiol Res 2010. 59(3): 347-56. PMID: 19681651
- Chun KJ, Park YH, Kim JS, Jang Y, Kim JH, et al. Comparison of 5 different remifentanil strategies against myocardial ischemia-reperfusion injury. J Cardiothorac Vasc Anesth 2011. 25(6): 926-30. PMID: 21514843
- Qiao S, Mao X, Wang Y, Lei S, Liu Y, et al. Remifentanil Preconditioning Reduces Postischemic Myocardial Infarction and Improves Left Ventricular Performance via Activation of the Janus Activated Kinase-2/Signal Transducers and Activators of Transcription-3 Signal Pathway and Subsequent Inhibition of Glycogen Synthase Kinase-3beta in Rats. Crit Care Med 2016. 44(3): e131-45. PMID: 26468894
- Hou J, Wang H, Li X, Zhu Y. Remifentanil functions in the adaptive protection of cardiac function following ischemia. Exp Ther Med 2017. 13(4): 1514-1520. PMID: 28413502
- Lei S, Su W, Xia ZY, Wang Y, Zhou L, et al. Hyperglycemia-Induced Oxidative Stress Abrogates Remifentanil Preconditioning-Mediated Cardioprotection in Diabetic Rats by Impairing Caveolin-3-Modulated PI3K/Akt and JAK2/STAT3 Signaling. Oxid Med Cell Longev 2019. 2019: 9836302. PMID: 31583053
- Zhang Y, Irwin MG, Wong TM. Remifentanil preconditioning protects against ischemic injury in the intact rat heart. Anesthesiology 2004. 101(4): 918-23. PMID: 15448525
- Zhang TZ, Zhou J, Jin Q, Sun YJ, Diao YG, et al. Protective effects of remifentanil preconditioning on cerebral injury during pump-assisted coronary artery bypass graft. Genet Mol Res 2014. 13(3): 7658-65. PMID: 25299079
- Schmid FX, Floerchinger B, Vudattu NK, Eissner G, Haubitz M, et al. Direct evidence of endothelial injury during cardiopulmonary bypass by demonstration of circulating endothelial cells. Perfusion 2006. 21(3): 133-7. PMID: 16817285
- Zhou T, Chuang CC, Zuo L. Molecular Characterization of Reactive Oxygen Species in Myocardial Ischemia-Reperfusion Injury. Biomed Res Int 2015. 2015: 864946. PMID: 26509170
- Skrabal CA, Choi YH, Kaminski A, Steiner M, Kundt G, et al. Circulating endothelial cells demonstrate an attenuation of endothelial damage by minimizing the extracorporeal circulation. J Thorac Cardiovasc Surg 2006. 132(2): 291-6. PMID: 16872952
- Woywodt A, Blann AD, Kirsch T, Erdbruegger U, Banzet N, et al. Isolation and enumeration of circulating endothelial cells by immunomagnetic isolation: proposal of a definition and a consensus protocol. J Thromb Haemost 2006. 4(3): 671-7. PMID: 16460450
- Lee KW, Lip GY, Tayebjee M, Foster W, Blann AD. Circulating endothelial cells, von Willebrand factor, interleukin-6, and prognosis in patients with acute coronary syndromes. Blood 2005. 105(2): 526-32. PMID: 15374879
- Sabatier F, Camoin-Jau L, Anfosso F, Sampol J, Dignat-George F. Circulating endothelial cells, microparticles and progenitors: key players towards the definition of vascular competence. J Cell Mol Med 2009. 13(3): 454-71. PMID: 19379144
- Woywodt A, Bahlmann FH, De Groot K, Haller H, Haubitz M. Circulating endothelial cells: life, death, detachment and repair of the endothelial cell layer. Nephrol Dial Transplant, 2002. 17(10): 1728-30. PMID: 12270976
- Videira RL, Cruz JR. Remifentanil in the clinical practice. Rev Bras Anestesiol, 2004. 54(1): 114-28. PMID: 19471719
- Hoke JF, Shlugman D, Dershwitz M, Michałowski P, Malthouse-Dufore S, et al. Pharmacokinetics and pharmacodynamics of remifentanil in persons with renal failure compared with healthy volunteers. Anesthesiology, 1997. 87(3): 533-41. PMID: 9316957
- Egan TD. Remifentanil pharmacokinetics and pharmacodynamics. A preliminary appraisal. Clin Pharmacokinet, 1995. 29(2): 80-94. PMID: 7586903