Translational Perioperative and Pain Medicine (ISSN: 2330-4871)
ARTICLE DOI: 10.31480/2330-4871/101
CASE REPORT OPEN ACCESS
Management Considerations in Complicated Peripartum Cardiomyopathy
Linda He, BA, MS1, Azfar Niazi, MD2 and Sabry Ayad, MD, MBA2*
1Eastern Virginia Medical School, Norfolk, VA, USA
2Outcomes Research Department, Anesthesiology Institute, Cleveland Clinic, Fairview Hospital, Cleveland, OH, USA
Sabry Ayad, MD, MBA, Professor of Anesthesiology, Outcomes Research Department, Anesthesiology Institute, Cleveland Clinic, Fairview Hospital, Cleveland Clinic Lerner College of Medicine, Case Western Reserve University, 18101 Lorain Ave., Cleveland OH 44111, USA, Tel: 216-476-7052, E-mail: saayad@ccf.org.
Editor: Yun Xia, MD, Professor, Department of Anesthesiology, The Ohio State University, N411 Doan Hall 410 West Tenth Avenue, Columbus, Ohio 43210-1228, United States, Email: yun.xia@osumc.ed
Received: August 07, 2019 | Accepted: September 02, 2019| Published: September 13, 2019
Citation: He L, Niazi A, Ayad S. Management Considerations in Complicated Peripartum Cardiomyopathy. Transl Perioper & Pain Med 2019; 6 (4):136-140
Abstract
We report a case of peripartum cardiomyopathy (PPCM) in an advanced maternal age (AMA), obese female with multiple risk factors. Early diagnosis and adequate treatment reduce significant patient mortality associated with this condition. In patients with multiple risk factors who present with symptoms of normal pregnancies, PPCM should be ruled out by echocardiogram and N-terminal pro b-type natriuretic peptide (NT-proBNP) quantification. Over a six-month period, the patient's left heart systolic function recovered slowly, although she was clinically asymptomatic. Because of her persistently low ejection fraction (EF) at the three-month follow-up, we initiated the use of sacubitril/valsartan, which induced cardiac recovery within three weeks. While sacubitril/valsartan has been proven to improve outcomes in heart failure patients, its use should proceed with caution in peripartum patients.
Keywords
Peripartum cardiomyopathy, Heart failure, Advanced maternal age, Angiotensin receptor neprilysin inhibitor
Introduction
Cardiomyopathy is a critical medical condition that involves damage to the heart muscles. Consequently, the heart cannot efficiently pump blood to the rest of the body. Peripartum cardiomyopathy (PPCM) is a form of cardiomyopathy most commonly seen in otherwise healthy women near the end of pregnancy or in the months following delivery. It occurs in approximately 1:1,149 to 4,000 peripartum females in the United States [1]. Left ventricular (LV) dysfunction characterized by EF less than 45% is often one of the criteria for diagnosis [1,2]. PPCM can be associated with or without preeclampsia/eclampsia. Some cases suggest that preeclampsia can cause PPCM [2]. The important risk factors include AMA, obesity, preeclampsia, history of cardiac disease, familial history, and multiple pregnancies [3].
Case Presentation
The exemption from the Institutional Review Board of Cleveland Clinic and consent from the patient were obtained for our case. A 42-year-old obese (body mass index 37.6 kg/m2) female at 38w2d, G2P1 with past medical history significant for gestational diabetes mellitus, presented with a three-day history of shortness of breath, orthopnea, chest tightness, tachycardia, headache, and swollen feet. She denied fever, vision changes, and right upper abdominal pain. No history of cardiac diseases or chronic hypertension was reported. Her vital signs included blood pressure (BP) of 145/98 mmHg, respiratory rate of 30 breaths per minute, heart rate of 150 beats per minute (bpm), and blood oxygen saturation (SpO2) of 98% on 10 liters per minute of oxygen through a non-rebreather mask. She was afebrile. Physical exam revealed loud S3 gallop, bilateral basilar rhonchi, and bilateral feet and ankle edema. Fetal monitoring was normal with a heart rate of 125 bpm. Laboratory results showed a white blood cell count of 12.8 k/µL, urine protein of 74 mg/dl, NT-proBNP of 1017 pg/ml, mildly elevated aspartate transaminase of 45 U/L and alanine transaminase of 30 U/L, and normal troponin. Electrocardiogram reported sinus tachycardia with borderline LV hypertrophy and frequent premature ventricular contractions (PVCs). Chest X-ray showed bilateral basilar infiltration. Echocardiogram revealed dilated LV with severely decreased function, EF of 25%, diffuse LV hypokinesia, and normal right ventricle function (Figure 1 and Figure 2). The patient started contractions and labor analgesia was administered through the epidural catheter; BP was noted to be 118/62 mmHg. Coagulation profile was normal. She was admitted to the intensive care unit (ICU) and Swan Ganz catheter, central, and arterial lines were inserted to monitor hemodynamics and heart function. Although our patient's clinical symptoms may lead us to consider preeclampsia, we ruled out preeclampsia because her labs did not meet criteria for diagnosis. Our patient was diagnosed with PPCM, for which she was placed on intravenous (IV) furosemide, tocolytic medication, and digoxin. Tocolytic medication was given to delay delivery until heart function improved, as the patient was unable to lie flat and EF was very low.
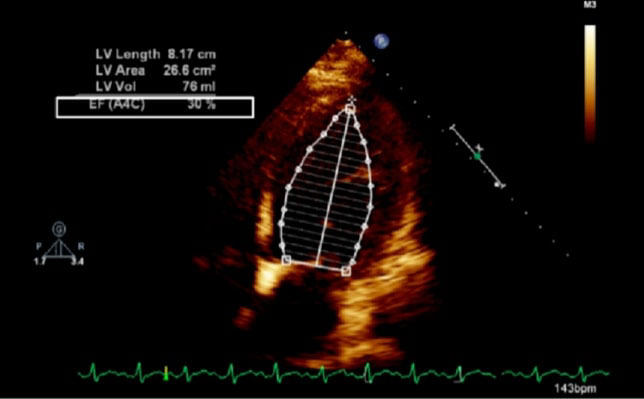
Figure 1: Echocardiogram of apical four chamber view of the heart showing dilated left ventricle and reduced ejection fraction; LV: left ventricle, EF: ejection fraction, A4C: apical four chamber, Vol: volume.
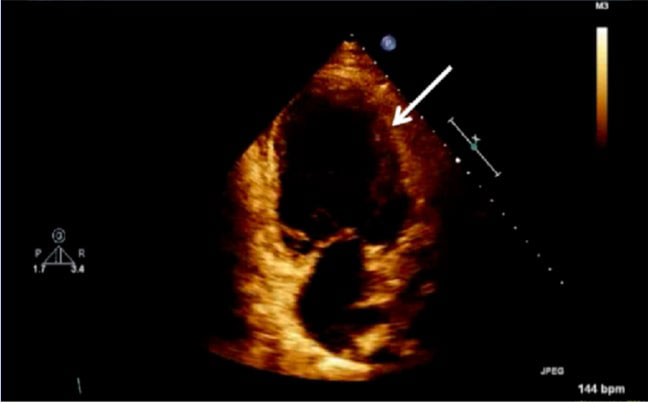
Figure 2: Echocardiogram of apical four chamber view of the heart, depicting left ventricular wall hypertrophy (white arrow).
The patient was started on a two-day treatment of 1) Diuretic, 2) Strict oral/ IV intake, 3) Digoxin, 4) Hydralazine, 5) Oxygen, and 6) Anticoagulation. Her symptoms improved, with a respiratory rate of 20, on four liters of oxygen through a nasal cannula, and heart rate of 120 bpm. Given her history of breech vaginal delivery, risk of shoulder dystocia, and current macrosomia, she underwent cesarean section and bilateral tubal ligation under epidural anesthesia without any anesthetic and operative complications two days later. A healthy infant with Apgar score of nine was delivered. The patient was stable at the end of the procedure. She was closely monitored and treated in the surgical ICU for PPCM postoperatively. A beta-blocker and an ACE inhibitor, captopril, were added to the current treatment. Two days postoperatively, although the patient improved clinically with heart rate of 95 bpm and SpO2 of 98% on room air, chest X-ray showed persistent pulmonary edema and cardiomegaly. On postoperative day four, our patient became more diaphoretic and short of breath, with sinus tachycardia, S3 gallop, and slight jugular venous distention. She was placed on IV furosemide and received increased doses of captopril and other medications. Consequently, she made significant progress in her course with isolated PVCs, a softer S3 gallop, and improved symptoms.
From postoperative days five to eight, the patient improved clinically and was ambulatory despite severely impaired LV systolic function and EF of 25%. She was then discharged home on digoxin, carvedilol, captopril, hydralazine, furosemide and spironolactone.
At the three-month follow-up, the patient was asymptomatic; however, decreased LV systolic function and low EF of 25% were reported. Lab results indicated high NT-ProBNP of 483 pg/mL. Hydralazine was stopped and the patient was started on sacubitril/valsartan. The patient had not been breastfeeding during this period. Echocardiogram performed at the six-month follow-up revealed EF of 35%, moderately dilated LV, and normal right ventricle. The patient reported feeling much better on sacubitril/valsartan and her cardiac function showed gradual improvement after initiating the new regimen.
Discussion
PPCM is a relatively rare disease that affects about 1:1,149 to 4,000 peripartum females in the United States and has a high mortality rate of 3.3 to 32% [1,2,4-6]. Early and precise intervention is crucial in managing patients with multiple high-risk factors. Our patient with a three-day history of shortness of breath, palpitation, headache, and swollen lower extremities was suspected to have PPCM. Lab investigations were immediately initiated. Echocardiography findings of severely depressed LV systolic function with EF of 25%, and lab report of significantly high NT-proBNP of 1017 pg/ml, helped us initiate treatment for PPCM. Elevated levels of NT-proBNP indicate ventricular response to increased preload and LV size associated with systolic heart failure. Normal NT-proBNP levels should be less than 300 pg/ml in patients who present with acute and decompensated heart failure [7]. Although NT-proBNP is not specific to distinguish between pregnancy-related conditions such as preeclampsia and heart failure, its levels are much higher in PPCM patients when compared to healthy peripartum counterparts [8-10]. Because of our timely and adequate management, the patient improved enough clinically to undergo cesarean section. She tolerated the anesthesia and procedure well with good postpartum recovery.
This patient had multiple risk factors- obesity, high BP, AMA, and preeclampsia -which prompted the clinical diagnosis of PPCM [11]. Patients with known cardiac disease and associated risk factors for developing PPCM should be assessed and counseled before conception or early during pregnancy to prevent onset [12]. However, when PPCM is suspected from the patient's clinical presentation, immediate instigation of labs and an echocardiogram should be performed to confirm the diagnosis and begin management. Although our patient did not reach the clinical criteria to diagnose preeclampsia, the symptoms of headache, rapid weight gain (10 lb in a week), edema, proteinuria, elevation of hepatic enzymes with mild elevation of BP, should alert the obstetrician to consider preeclampsia. However, we ruled out preeclampsia because of the relatively mild elevations of hepatic enzyme, BP, and mild proteinuria. Relatively lower BP, in this case, is secondary to congestive heart failure.
The management of PPCM is the task for a multidisciplinary team of cardiologists, ob¬stetricians, intensivists, and anesthesiologists [1]. The Swan Ganz catheter was used to closely monitor pressure changes in the left atrium associated with heart failure. It is the standard protocol in our facility to manage decompensated heart failure. The initial epidural catheter was placed to reduce the patient's pain from contractions and relieve her hemodynamic stress. Because of her unstable BP, arrhythmias, pulmonary edema, class IV heart function, and significant hemodynamic changes during labor, hemodynamic monitoring was critical to predict rapid deterioration of heart failure [13-15]. Although delivery can reduce pregnancy-induced hemodynamic stress, it cannot restore heart function immediately [3]. The patient's cardiac decompensation improved with IV furosemide and an increased dose of captopril.
The decision of timing and mode of delivery was based on the patient's clinical status. Termination of pregnancy irrespective of gestational age may be considered in patients with hemodynamic instability because this may improve cardiac function and enable effec¬tive medical treatment [3,12]. A cesarean section was planned and performed because of the patient's critical and severely depressed heart function and previous history of breech and risk of shoulder dystocia. The delivery was delayed for two days to allow cardiac function to improve. Some case reports showed occurrences of cardiogenic shock during cesarean section [3,16]. Our management was comprehensive, considered, and avoided aggressive exacerbation of cardiac function by cesarean section. A follow-up echocardiogram reported no improvement of the patient's left heart function (EF remained low at 25% although the patient felt significantly better). Because the majority of deaths occur within the first three to six months after diagnosis, the patient should be medicated for at least six months [17]. A beta-blocker was administered to reduce mortality associated with cardiomyopathy.
At three months postpartum, although the patient was asymptomatic, her LV systolic function did not recover, with an EF of only 25%. Complete recovery, reported in 23% to 72% of PPCM patients, may be contributed to early diagnosis and adequate management of heart failure [2,12,17].
Sacubitril/valsartan, an Angiotensin Receptor Neprilysin Inhibitor (ARNI), is effective in reducing mortality and hospitalization associated with heart failure, as shown in the PARADIGM-HF clinical trials; however, there is little literature on its use and effects in peripartum and postpartum patients [18,19]. Based on the properties of its drug class and pharmacokinetics, sacubitril/valsartan is generally contraindicated or should be used with caution in this patient population [19,20]. Its administration, therefore, was considered on a case-by-case basis. We decided to give our patient sacubitril/valsartan, a practice not commonly seen for peripartum and postpartum patients, because of her persistently low EF that showed no improvement following three months of the original treatment. Soon after initiating the new therapy, our patient's cardiac function was recovering, with EF of 35% [21]. Physician discretion on the use of an ARNI in our postpartum patient, paired with timely diagnosis and effective treatment modalities, decreased patient mortality in PPCM.
Conclusions
PPCM confers high mortality in peripartum and postpartum women who may have an unremarkable medical history for cardiovascular disease. Early recognition by echocardiogram and NT-proBNP quantification and immediate institution of therapy increase the odds of low morbidity in such a condition. We were able to rule out preeclampsia, a common complication associated with pregnancy, because of the mild elevations of hepatic enzymes, BP, and mild proteinuria. Acute congestive heart failure attributed to the lower BP in our case. While the clinical presentation of PPCM may be similar to the symptoms of normal pregnancy, our comprehensive diagnosis and management were possible because of our team collaboration, allowing for a favorable patient outcome.
References
- Sliwa K, Böhm M: Incidence and prevalence of pregnancy-related heart disease. Cardiovasc Res. 2014, 15:554-60. 10.1093/cvr/cvu012
- Kim M-J, Shin M-S: Practical management of peripartum cardiomyopathy. Korean J Intern Med. 2017, 32:393-403. 10.3904/kjim.2016.360
- Sliwa K, Hilfiker-Kleiner D, Petrie MC, et al.: Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail. 2010, 12:767-78. 10.1093/eurjhf/hfq120
- Fett JD, Christie LG, Carraway RD, Murphy JG: Five-year prospective study of the incidence and prognosis of peripartum cardiomyopathy at a single institution. Mayo Clin Proc. 2005, 80:1602-6. 10.4065/80.12.1602
- Brar SS, Khan SS, Sandhu GK, et al.: Incidence, mortality, and racial differences in peripartum cardiomyopathy. Am J Cardiol. 2007, 15:302-4. 10.1016/j.amjcard.2007.02.092
- Duran N, Günes H, Duran I, Biteker M, Ozkan M: Predictors of prognosis in patients with peripartum cardiomyopathy. Int J Gynaecol Obstet. 2008, 101:137-40. 10.1016/j.ijgo.2007.11.007
- McCullough PA, Kluger AY: Interpreting the wide range of NT-proBNP concentrations in clinical decision making. J Am Coll Cardiol. 2018, 71:1201-1203. 10.1016/j.jacc.2018.01.056
- Haghikia A, Podewski E, Libhaber E, et al.: Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol. 2013, 108:366. 10.1007/s00395-013-0366-9
- Junus K, Wikström A-K, Larsson A, Olovsson M: Placental expression of proBNP/NT-proBNP and plasma levels of NT-proBNP in early- and late-onset preeclampsia. Am J Hypertens. 2014, 27:1225-30. 10.1093/ajh/hpu033
- Forster O, Hilfiker-Kleiner D, Ansari AA, et al.: Reversal of IFN-gamma, oxLDL and prolactin serum levels correlate with clinical improvement in patients with peripartum cardiomyopathy. Eur J Heart Fail. 2008, 10:861-8. 10.1016/j.ejheart.2008.07.005
- Shah T, Ather S, Bavishi C, Bambhroliya A, Ma T, Bozkurt B: Peripartum cardiomyopathy: a contemporary review. Methodist Debakey Cardiovasc J. 2013, 9:38-43. PMC3600883
- Ruys TPE, Cornette J, Roos-Hesselink JW: Pregnancy and delivery in cardiac disease. J Cardiol. 2013, 61:107-12. 10.1016/j.jjcc.2012.11.001
- Midei MG, DeMent SH, Feldman AM, Hutchins GM, Baughman KL: Peripartum myocarditis and cardiomyopathy. Circulation. 1990, 81:922-8. 10.1161/01.CIR.81.3.922
- McMurray JJV, Adamopoulos S, Anker SD, et al.: ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur J Heart Fail. 2012, 14:803-69. 10.1093/eurjhf/hfs105
- Dinic V, Markovic D, Savic N, Kutlesic M, Jankovic RJ: Peripartum cardiomyopathy in intensive care unit: an update. Front Med. 2015, 2:82. 10.3389/fmed.2015.00082
- Hamdan R, Nassar P, Zein A, Issa M, Mansour H, Saab M: Peripartum cardiomyopathy, place of drug therapy, assist devices, and outcome after left ventricular assistance. J Crit Care. 2017, 37:185-8. 10.1016/j.jcrc.2016.09.028
- McNamara DM, Elkayam U, Alharethi R, et al.: Clinical outcomes for peripartum cardiomyopathy in North America: results of the IPAC study (Investigations of Pregnancy-Associated Cardiomyopathy). J Am Coll Cardiol. 2015, 66:905-14. 10.1016/j.jacc.2015.06.1309
- McMurray JJV, Packer M, Desai AS, et al.: Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014; 371:993-1004. 10.1056/NEJMoa1409077
- Kearney L, Wright P, Fhadil S, et al.: Postpartum cardiomyopathy and considerations for breastfeeding. Card Fail Rev. 2018; 4:112–118. 10.15420/cfr.2018.21.2
- Arrigo M, Blet A, Mebazaa A: Bromocriptine for the treatment of peripartum cardiomyopathy: welcome on BOARD. Eur Heart J. 2017, 38:2680–2682. 10.1093/eurheartj/ehx428
- Arnaout R, Nah G, Marcus G, et al: Pregnancy complications and premature cardiovascular events among 1.6 million California pregnancies. Open Heart. 2019,6:e000927. 10.1136/openhrt-2018-000927