Translational Perioperative and Pain Medicine (ISSN: 2330-4871)
ARTICLE DOI: 10.31480/2330-4871/093
RESEARCH ARTICLE OPEN ACCESS
Spinal Cord Stimulation for Failed Back Surgery Syndrome -- Patient Selection Considerations
Nicole Palmer, MD1, Zhonghui Guan, MD2 and Nu Cindy Chai, MD2*
1University of California San Francisco Medical School, San Francisco, CA, USA
2Division of Pain Medicine, Department of Anesthesia and Perioperative Care, University of California, San Francisco, CA, USA
Nu Cindy Chai, MD, University of California, San Francisco, 2255 Post Street, San Francisco, CA 94115, USA, Tel : 415-885-7246, Fax : 415-885-7575, E-mail: nu.chai@ucsf.edu
Editor: Renyu Liu, MD; PhD, Associate Professor, Department of Anesthesiology and Critical Care, Perelman School of Medicine at the University of Pennsylvania, 336 John Morgan building, 3620 Hamilton Walk, Philadelphia, PA 19104, USA, E-mail: liur@uphs.upenn.edu
Received: April 15, 2019 | Accepted: June 14, 2019| Published: June 21, 2019
Citation: Palmer N, Guan Z, Chai NC. Spinal Cord Stimulation for Failed Back Surgery Syndrome -- Patient Selection Considerations. Transl Perioper & Pain Med 2019; 6(3):81-90.
Abstract
Background
Failed back surgery syndrome (FBSS) refers to the condition where persistent pain is experienced by patients following back surgery. This condition is historically difficult to treat. Spinal cord stimulation (SCS) and its recent technical advances have opened the door to a promising treatment option for FBSS. However, critical appraisal of supporting and refuting data is necessary to identify the best patient population for this treatment modality.
Methods
In this systematic review, we review randomized controlled studies and cohort studies with matched controls to synthesize the data on the overall efficacy of spinal cord stimulation for FBSS. We further identify available data on outcome measurements based on working status, psychological status, smoking, sex, and race to provide insight on patient selection and identify needs for further research.
Results
The literature search identified 34 publications, of which 23 were excluded due to duplication and inclusion/exclusion criteria, yielding a total of 11 publications for review. Seven out of eleven studies reviewed had sources of potential funding or affiliation bias. Three out of 4 studies with radiating leg pain relief as their primary outcome showed statistically significant improvement with SCS treatment, while 2 out of 5 studies with mixed radiating leg pain and axial back pain as the primary outcome showed statistically significant improvement with SCS. All randomized controlled trials that included functional status and quality of life outcome measures showed improvement after SCS, though scales utilized in each study varied. Six studies included work status as a patient descriptor with only three reporting inclusion of workers' compensation patients. There was limited data on the effect of psychological status, smoking, sex or race on SCS outcomes based on the studies reviewed.
Conclusions
Evidence for the efficacy of SCS in FBSS is accumulating, with most studies demonstrating its efficacy especially for those patients with leg pain as the predominant symptom. However, a significant weakness in the current data includes potential bias based on the funding source for most studies. Additionally, it is clear that SCS provides short-term benefit, yet there is no solid evidence that SCS provides any benefit beyond two years of implantation. Another major concern is the significant placebo effect, which makes the true therapeutic response difficult to judge. Further, it is increasingly important to focus future studies on refining patient populations to those that may best respond to both SCS therapy in general, as well as specific stimulation techniques.
Introduction
Neuromodulation as a concept to treat medical ailments has been documented for centuries [1]. Spinal cord stimulation (SCS) as a type of neuromodulation to treat pain was first developed by Dr. Norman Shealy more than 50 years ago when he implanted the first stimulator device in a cancer pain patient [2]. This is the direct translational practice of the gate control theory, which hypothesized that the activation of A fiber mediated touch sensation in the spinal cord can inhibit C fiber mediated pain sensation [3]. However, the true mechanism of SCS is much more complex and has not been fully elucidated. For example, SCS directly inhibits spinothalamic pain pathways and affects upstream supraspinal inhibitory pathways to reduce pain. Further, it has been shown that SCS can change peripheral blood flow and may therefore affect pain by vasodilation and improvement of blood flow in specific cases [4]. Over the past several decades, there has been tremendous progress in both technological advances of this modality as well as research efforts to refine its target population and to validate its efficacy. In the face of the opioid epidemic, this technology has further gained traction and public attention, especially in the population of patients that has traditionally been difficult to treat.
One chronic pain condition that has seen tremendous research and positive outcomes with SCS is failed back surgery syndrome. Also called post-laminectomy syndrome, it describes the condition where persistent pain is experienced by patients following back surgery. The incidence of this condition has been found to be between 10-40% after lumbar back surgery [5,6]. The etiology of the persistent pain varies, including persistent radicular pain from long-term nerve root injury not improved by surgery, re-stenosis of neuroforamens or central canals, facet disease after lumbar fixation, or pain from a persistent surgical scar. These patients typically do not experience much improvement with multiple conservative therapies such as oral medications, physical therapy, and various injections even prior to the back surgery. In these cases, persistent pain after back surgery can be exponentially difficult to manage.
In this paper, we will systematically review the scientific evidence behind spinal cord stimulation with a focus on patient selection considerations for the indication of failed back surgery syndrome.
Methods
A) Search Strategy
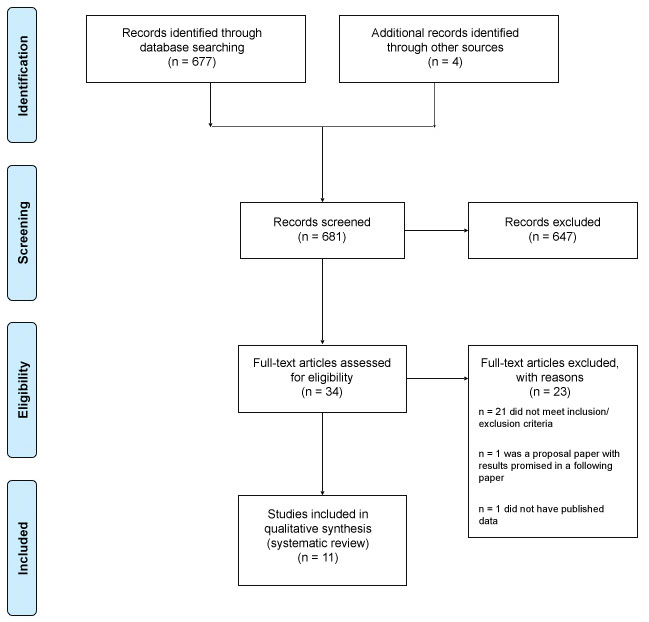
A literature search was conducted by the authors to identify randomized controlled trials and matched cohort-control studies analyzing the efficacy of SCS for the indication of FBSS or post-laminectomy syndrome. The search was conducted using PubMed, Ovid and MEDLINE. MeSH terms included ["failed back surgery syndrome" AND neuromodulation], [neuromodulation AND FBSS], ["failed back surgery syndrome" AND "spinal cord stimulation"], [FBSS AND "spinal cord stimulation"], and ["spinal cord stimulation" AND "post-laminectomy syndrome"]. Studies published between 1991 and 2018 were included. A total of 677 studies were identified through a PubMed search. Four more studies were identified through the citations of relevant aforementioned studies. The studies were further narrowed by screening for clinical studies and removing duplicates. After applying the stated inclusion and exclusion criteria and removing two studies with missing data, 11 studies were ultimately included in our analysis (Figure 1).
Figure 1:
B) Selection Criteria
All RCTs and cohort-control studies were assessed for inclusion in this review by two authors. Studies without inclusion of FBSS patients were excluded, as were studies without controls. The Jadad Scale [7] was used to assess the quality of each study.
Inclusion Criteria
1. Randomized controlled studies OR cohort studies with matching control.
2. Spinal cord stimulation studies for the indication of pain in post-laminectomy syndrome or failed-back surgery syndrome.
3. Studies with patient-related clinical primary or secondary outcomes.
Exclusion Criteria
1. Retrospective or non-controlled studies.
2. Spinal cord stimulation studies for the indication of other pain conditions or for non-pain conditions.
3. Studies that include other pain diagnoses in addition to FBSS but do not disaggregate outcome data by patient diagnosis.
4. Spinal cord stimulation studies that did not assess pain scores or measures of functional pain changes.
C) Outcomes Measured
Our measured outcomes were chosen according to common patient-related clinical outcomes reported in the reviewed studies as well as outcomes used in clinical decision making between the patient and physician. They include changes in:
- Perceived pain
Analgesic use
- Quality of life
- Quality of life
- Patient satisfaction
D) Summary Measures and Synthesis of Results
Each study used its own outcomes scales. As a result, there is a heterogeneous landscape of quantified outcomes across all RCTs and cohort-control studies for SCS in FBSS patients. Consequently, in our analysis we could not combine data to generate consolidated numerical evidence. The data is therefore presented individually and then synthesized.
Results
The Literature search identified 57 publications, of which 46 were excluded due to duplications and inclusion/exclusion criteria, yielding a total of 11 publications for review.
Summary of Efficacy
Pain
Of the 11 studies selected for review, five studies focused on axial or radiating pain relief as their primary outcome [8,12,15-17]. Four studies included only radiating pain relief as their primary outcome, and two studies reported only axial pain relief as their primary outcome [9,10,11,13,14,18].
Three out of 4 studies with radiating leg pain relief as their primary outcome showed statistically significant improvement with SCS treatment [10,13,14]. Kumar, et al.'s PROCESS study looked at the difference in leg pain relief between SCS and conventional medical management (CMM) with a follow-up period of 6 months and 2 years [10,14]. At 6 months, they found that 48% of the SCS cohort had achieved at least 50% leg pain reduction, while only 9% of the CMM cohort achieved the same goal (p < 0.0001) [14]. At 24 months, they found that 37% of patients in the SCS cohort maintained at least 50% leg pain reduction compared to 2% in the CMM group [10]. Schu, et al. compared the efficacy of burst stimulation, 500 Hz tonic stimulation and placebo [13]. At 3 weeks follow-up, they reported a statistically significant improvement in mean NRS score for burst stimulation (5.6 to 4.7) that was superior to other groups (500 Hz tonic stimulation (mean NRS = 7.10) and placebo (mean NRS = 8.3), p < 0.05). Finally, Turner, et al compared 3 different cohorts of workers' compensation patients - those randomized to SCS, pain clinic, or usual care [11]. In this study, success for each modality was defined as achieving all of the following: 1. at least 50% pain reduction, 2. at least a 2-point reduction on the Roland-Morris Disability Questionnaire score, and 3. reduction in daily opioid use. At 6 months follow-up, they concluded that SCS was more successful than the other modalities (SCS: 18% of cohort experienced success, Pain clinic: 5%, Usual care: 3%); however, at 12 and 24 months they reported that there was no statistically significant difference between groups.
Pain relief with respect to axial pain was the primary outcome in two studies [9,18]. North, et al. in June 2005, compared dual electrode to single electrode placement and showed that at the 2.3 year post-implantation follow-up, 48% of the study population had at least 50% pain relief with no difference between the number of electrodes used [18]. Statistical significance was not reported. Al-Kaisy, et al. compared various levels of high frequency stimulation and sham [9]. Baseline mean VAS for back pain was recorded as 7.75, while mean scores for sham, 1200 Hz, 3030 Hz, and 5882 Hz were 4.83, 4.51, 4.57, and 3.22, respectively. The study demonstrated statistically significant reduction in VAS score across all groups (sham, 1200 Hz, 3030 Hz, and 5882 Hz) from baseline, p < 0.001, with only one frequency (5882 Hz) in pair-wise analysis being superior to sham. Notably, this study revealed a significant placebo component to high frequency stimulation, complicating prior study results and necessitating further research into this area.
Two out of five studies with axial or radiating pain relief as their primary outcome showed statistically significant improvement with SCS treatment [8,16]; one study showed clinically significant improvement without statistical significance [17]; and, two studies did not report significance for the final follow-up, though one provided evidence for significant pain relief at an earlier follow-up interval [12,15]. The follow-up period for these studies ranged from 2 weeks to 3 years. North, et al compared SCS implantation in FBSS patients to standard re-operation [8]. At a 3 year follow-up, they found that 47% of post-implantation SCS cohort patients achieved 50% pain reduction and were satisfied with their results, compared with 12% of the re-operation cohort (p < 0.01). In a later study in 2005, North, et al looked at the efficacy of percutaneous vs. laminectomy electrode placement [17]. At the 1.9 year follow-up, 83% of patients with laminectomy electrodes and 42% of patients with percutaneous electrodes had at least 50% pain relief (p < 0.05). At 2.9 years follow-up, they found no statistically significant difference in pain relief between groups, but showed at least a 50% pain reduction in 42% of laminectomy implants and 25% of percutaneous implants (authors did not provide a p-value, but noted this finding to be statistically insignificant). Van Havenbergh, et al studied the efficacy of 500 Hz vs. 1000 Hz burst stimulation for pain relief and found no significant difference between the two frequencies for back pain (p = 0.90), limb pain (p = 0.76), or general pain (p = 0.55); however, they did show an overall Visual Analogue Scale (VAS) reduction to 5/10 from baseline - though, notably, baseline was not reported and a p-value was not given [12]. Van Gorp, et al. looked at patients who had adequate leg pain relief, but inadequate back pain relief with SCS, and added peripheral nerve field stimulation (PNFS) to observe the effects on back pain [16]. At 12 months follow-up, they concluded that PNFS plus SCS provides superior back pain relief than SCS alone (p < 0.001) - on the VAS scale, back pain with SCS reduced from a mean of 73.9 to 68.3 (p < 0.001), and leg pain reduced from a mean of 71.8 to 12.9 (p < 0.001) at 3 months follow-up (prior to adding PNFS to the SCS treatment). De Andres, et al. compared conventional SCS to high frequency SCS (HFSCS) and noted a clinically significant reduction in pain for both groups (20-25% reduction in average Numerical Rating Scale (NRS) score at 1 year) [15]. This was not statistically significant (p = 0.560) and there was no significant difference between groups (p = 0.11).
Analgesic use
Seven studies included changes in analgesic use as a secondary outcome [8,10-11,14,16-18]. Two studies reported a statistically significant improvement in overall analgesic use: North, et al reported significantly lower opioid use within the SCS group than the re-operation group (p = 0.025), while van Gorp, et al reported a decrease in Medication Quantification Scale (MQS) from 14.0 to 11.4 within the SCS and PFNS group (p = 0.017) without mention of the effects of SCS alone [8,16]. One study showed significant reduction only in anticonvulsant use (odds ratio = 0.35, p = 0.02) at 6mo follow-up [14]. Other drug categories in the study (opioids, NSAIDs, antidepressants) demonstrated similar downward trends. At 24 months follow-up, opioids and anticonvulsants continued to trend downward, though this was not statistically significant [10]. Turner, et al. results suggested an initial statistically insignificant improvement that was lost by 12 months [11]. In North, et al's study, the number of electrodes placed did not significantly change analgesic use (41% decreased use, 53% increased use) [18]; however, electrode placement did produce change - 75% of patients with laminectomy vs. 33% with percutaneous placement achieved a reduction in prescription analgesic use [17]. The level of the reduction was not mentioned.
Functional Change
Nine studies included functional change as a secondary outcome [8,10,11,13-18]. change to "One study reported reduction of Oswestry Disability Index (ODI) from 56.1 to 44.9 (p < 0.001) at 6 months and 59 to 47 (p < 0.0002) at 24 months with SCS compared to conventional medicine." [10]. ODI scores reduced similarly with SCS burst treatment in a study comparing placebo, burst, and 500 Hz stimulation (baseline 22.3, burst 19.2) [13]. Comparing re-operation to SCS, there was a greater net loss of function in patients who underwent re-operation, while SCS patients always experienced a net functional gain [8]. In a study comparing single to dual electrode placement, most study participants across both groups did not experience impairment in activities of daily living; however, 53% of the total study population experienced decreased strength or coordination and 12% experienced decreased bladder or bowel control (p-value not provided) [18]. One study comparing laminectomy to percutaneous electrode placement reported that laminectomy electrodes supported greater net functional improvement over percutaneous electrodes [17]. No statistically significant difference in function was found when comparing SCS to conventional treatment options among workers' compensation patients at 12 and 24 months follow-up [11]. In a comparison between conventional stimulation and high frequency stimulation, both showed significant reduction in ODI with no between-group differences [15]. Van Gorp, et al. did not report on ODI outcomes for SCS alone vs. baseline [16].
Quality of Life
Six studies included changes in quality of life as a secondary outcome; five of the six used validated tools: Short Form-36 (SF-36), Short Form-12 (SF-12), EuroQoL five dimensional (EQ-5D) index, and the Pain Vigilance and Awareness Questionnaire (PVAQ) [10,12,14-16,18]. Four studies reported a statistically significant improvement in overall quality of life [10,14-16]. The PROCESS study showed increased quality of life in the SCS group compared to the conventional medical management group at 6 months (SF-36: 7 of 8 health-related quality of life measures were enhanced, p < 0.02; EQ-5D: differential improvement from baseline of 0.23) and at 24 months (SF-36: 7 of 8 health-related quality of life measures were enhanced, p < 0.01; EQ-5D improved by about 0.30 from baseline, p < 0.0001) [10,14]. While van Gorp, et al. demonstrated the superior functional improvement of SCS with the addition of PFNS (as assessed by SF-36), they did not report data for SCS alone [16]. Van Havenbergh, et al. used SF-36 and PVAQ to report no significant difference in quality of life between 500 Hz burst stimulation and 1000 Hz burst stimulation; however, the authors did not provide a reference to baseline data [12]. De Andres showed that both conventional and HFSCS provided significant quality of life improvement in all domains, as per the SF-12 form [15].
Patient Satisfaction
Eight studies included patient satisfaction as a secondary outcome [8-10,13-17]. Six reported a favorable patient response toward SCS treatment, with two studies revealing that patients would prefer either undergoing SCS implantation again or undergoing the implantation in place of another back surgery if given the choice [8-10,13-17]. The 2007 Kumar study concluded that 66% of patients receiving SCS were satisfied with their pain relief, while 86% were satisfied with treatment overall (p < 0.001) [14]. In the 2008 Kumar, et al study, it was noted that 66% of patients receiving SCS were satisfied with pain relief, while 93% were satisfied with the treatment overall (p-value not reported) [10]. Schu, et al. reported that 80% of patients preferred burst stimulation above other forms of stimulation (p = 0.0004) [13]. Van Gorp, et al. reported mean Patient Global Impression of Change (PGIC) at 12 months as 3.3, indicating an impression of minimal to moderate recovery [16]. De Andres, et al. reported that both conventional stimulation and HFSCS had significant improvement in PGIC (increase of 0.64 and 0.96, respectively); but, they were not statistically different from each other [15]. Al-Kaisy, et al. reported that PGIC scores showed that most patients believed that 5882 Hz stimulation provided considerable improvement; however, among those who were very satisfied or somewhat satisfied with the therapy, there was no statistically significant difference between sham (63%), 1200 Hz (63%), 3030 Hz (75%), and 5882 Hz (75%) (p = 0.672) [9].
Patient Selection Consideration Overview
In this review, most studies focused on patient selections considerations such as patient sex, age, number of prior lumbar surgeries, time since last surgery, and location of pain. Our goal was to determine whether there is evidence for additional considerations for patient selection, specifically working status, psychological health, smoking status, sex and race. Six studies included working status and 3 studies included patients with worker's compensation [8,10,11,14,17,18]; 5 studies did not mention working status or worker's compensation. Six studies included psychological pre-testing for all patients and one study pre-screened only 25% of the SCS cohort [8,9,11,13-16]. Zero studies included information on patient race or smoking status.
Working Status
Six studies included work status as a patient descriptor with only three reporting inclusion of workers' compensation patients [8,10,11,14,17,18]. Comparing re-operation to SCS implantation, North, et al. in Jan 2005, found that there was no significant difference in a patient's ability to return to work [8]. North, et al. in Nov 2005, reported that two patients who pre-operatively were unable to work due to their pain returned to work post-operatively, while another patient moved from part-time to full-time post-operatively [17]. In looking at CMM vs. SCS and CMM, Kumar, et al. also found that there were no significant return-to-work differences between the two groups; however, it was noted that at 24 months follow-up 5 patients in the SCS group returned to work (4 of which had been out of work for a mean of > 2.5 years) while 3 patients stopped working without further explanation [10,14]. Turner, et al. analyzed the level of SCS benefit in workers' compensation patients in Washington State and found there is no evidence for statistically significant benefit overall [11]. Turner, et al. also concluded that there is not a significant benefit for improving pain or function in this population. All other studies did not comment on pain improvement in this specific patient subset.
Psychological Health
In this review, eight out of eleven studies definitively included mental health pre-screening [8-11,13-15]. In contrast, mental health was not mentioned in the three remaining studies [12,17,18]. Seven of these studies reported active and/or untreated psychiatric disorders as grounds for exclusion [8-10,13-15]. No studies compared psychiatric status to pain outcomes except Turner, et al, who found that patients with higher mental health screening scores (indicating healthier psychiatric status) had significantly better outcomes with respect to pain than those with lower scores [11]. Notably, Turner, et al. screened only 25% of patients in the SCS group and none in the Pain Clinic group.
Smoking Status
None of the studies in this review reported on the efficacy of SCS in relationship to smoking status.
Sex
All eleven studies reported baseline sex characteristics of participants; however, outcome data was not disaggregated according to sex in any study [8-18].
Race
None of the studies in this review reported on the efficacy of SCS in relationship to race.
Discussion
Since the first trials of spinal cord stimulation for chronic pain, there have been significant advancements in SCS delivery. Our understanding of its therapeutic capacity as well as its limitations has continued to evolve. The evidence found within the body of research investigated in this systematic review on spinal cord stimulation for FBSS showed that the results are mixed; but, they overall seem to suggest that SCS is likely efficacious for the short term relief of pain from FBSS. Based on the current available highest level evidence (ie randomized controlled studies vs. cohort studies of matched controls), pain relief from SCS is generally better than pain relief achieved with repeat back surgery or medical management. Pain relief is more likely achieved with SCS for those with leg pain as the predominant symptom versus those with axial back pain. In addition, a higher percentage of patients seem to report pain relief earlier in the follow up period (ie months after implant) compared to later (ie years after implant). This is especially important for further clarification, as at least one study reported a similar level of pain relief between high-frequency spinal cord stimulation and placebo [12].
Working Status
Work-related injuries impacting work status characterize an important subgroup of patients with low back pain. Some studies have suggested that those receiving Workers' Compensation experience worse pain outcomes overall regardless of treatment [19,20]. This has been challenged with evidence that it is specifically patient return-to-work expectations and the time required before returning to work that significantly affects pain levels [21]. These studies suggest that work status may be insufficient unto itself for understanding patient outcomes, calling for a more nuanced approach to patient selection within this category. Overall, the studies examined suggest that SCS treatment likely positively influences patients' ability to return to work. This is consistent with the data presented in a recent meta-analysis [22]. The Turner, et al. study [11] - which did not find significant pain relief with SCS compared to medical therapy in the workers' compensation population - had a few issues of note. First, it was a cohort study with matched controls instead of a randomized controlled study. Second, the population included in the study tended to have more severe pain and for longer periods of time than those of other studies, which makes it less generalizable. And lastly, patients who had failed SCS trials were eventually also included in the long term analysis of the data.
Psychological Health
The impact of mental health on pain treatment response has been well documented in the literature. Notably, in a 2009 review, psychological characteristics such as depression, anxiety, somatization, and poor coping were deemed as relevant predictors for poorer outcomes after device implantation [23]. A 2016 study added that the chosen outcomes in a study, such as functionality or pain catastrophizing, impact which psychiatric factors are important for screening in a study population [24]. Thus, screening for the psychiatric co-morbidities that correlate with the desired outcome measures is important for more accurate predictions of treatment outcome and is certainly an area that deserves further research. Overall, it is now standard of care in most practices to include psychological testing as a screening test for SCS implantation. Of note, the Turner, et al. study [11] included patients with lower scores showing worse psychological status, which may have contributed to the negative result of the study.
Smoking Status
Various studies have shown that smoking status impacts chronic pain treatment outcomes. Fishbain, et al. observed the impact of smoking status on a cohort of patients with chronic low back pain, citing that pain outcomes were worse among smokers regardless of follow-up time period [25]. Additionally, Hooten, et al. analyzed the effects of smoking on multidisciplinary pain rehabilitation program efficacy and found that smokers had worse outcomes with respect to some adjunctive modifiers of pain, notably pain catastrophizing and depression [26]. However, none of the studies included in this review provided subgroup analysis regarding the impact of smoking status on the success of SCS implant for FBSS. This remains a knowledge gap that is in need of further investigation.
Smoking Status
Various studies have shown that smoking status impacts chronic pain treatment outcomes. Fishbain, et al. observed the impact of smoking status on a cohort of patients with chronic low back pain, citing that pain outcomes were worse among smokers regardless of follow-up time period [25]. Additionally, Hooten, et al. analyzed the effects of smoking on multidisciplinary pain rehabilitation program efficacy and found that smokers had worse outcomes with respect to some adjunctive modifiers of pain, notably pain catastrophizing and depression [26]. However, none of the studies included in this review provided subgroup analysis regarding the impact of smoking status on the success of SCS implant for FBSS. This remains a knowledge gap that is in need of further investigation.
Sex
A comprehensive 2009 review by Fillingim, et al. looked at differences in the prevalence of pain between men and women in numerous forms and settings [27]. They concluded that of the most common forms of pain, women experience a higher prevalence and intensity of pain than men. With respect to analgesic response, a meta-analysis by Niesters, et al. reported that there is inconclusive evidence for differences in opioid response between men and women [28]. Yet, differences in non-analgesic treatment responses between sexes were noted in Kheog, et al's study, which observed significantly more pain and catastrophizing among post-treatment women after 3 months [29]. However, none of the studies included in this review provided subgroup analysis regarding the impact of sex on the success of SCS implant for FBSS. This remains a knowledge gap that is in need of further investigation.
Race
Race has routinely been included as a variable in contemporary scientific inquiry. To continue in this tradition the category was included in our review. However, the utility of race itself as a measure of analysis can be called into question when assessing responses to pain treatment. Race is a fluid and evolving sociopolitical category with limited biologic significance [30-33]. Racial groups themselves are heterogeneous making intra-race variability an important consideration for the accuracy and generalizability of race-based results [34]. And while racial disparities with respect to the epidemiology, access, and experience of pain have been reported in the literature, more recent studies have concluded that racial health disparities generally exist as consequences of racism, rather than race itself [35-39]. In a 2009 review on racial disparities in pain, Anderson, et al. concludes that studies should control for variables such as discrimination, immigration, the process of acculturation, health status, education, occupation, income, neighborhood socioeconomic level, and language of choice, among others [34]. Analyzing data from 13,777 patients, Reyes-Gibby, et al found that significant predictors for racial differences in pain severity included chronic disease, psychosocial distress, Medicaid insurance, and lower education level [40]. Thus, assessing a multitude of factors associated with the nuanced consequences of racism in its various forms (internalized, interpersonal, institutional [41]) rather than the imprecise and outdated categories of race would facilitate more accurate analyses of pain treatment outcomes. However, none of the studies included in this review provided subgroup analysis regarding the impact of these variables on the success of SCS for FBSS. This remains a knowledge gap that is in need of further investigation.
Limitations
Limitations for this systematic review include a low volume of studies available for review as well as the inability to consolidate data due to the heterogeneity of quantitative analysis tools between studies. Individual study limitations chiefly remain the inability to compare outcomes to a placebo group until the advent of more advanced delivery methods. Only 3 studies were of high quality as assessed by the Jadad scale [9,13,15]. Further, the patient characteristics assessed were not exhaustive and there are additional patient characteristics that can affect treatment outcomes that were not mentioned in this review.
Study Bias
Importantly, seven out of the eleven studies reviewed had sources of potential funding or affiliation bias [8-14]. Four studies used Medtronic devices and were simultaneously funded and/or managed by Medtronic [8-10,14]. Authors involved in the PROCESS study were also receiving financial reimbursements from Medtronic for consultancy work [10,14]. Two authors listed on the Schu, et al. paper received a research fellowship grant from, and served as consultants for, St. Jude Medical - the device manufacturer for their study [13]. Two authors for the Al-Kaisy [9] study received sponsorship and speaker fees from Medtronic, while two different authors on the same study are Medtronic employees. Van Havenbergh's study was not funded by St. Jude Medical; however, one of the authors is a St. Jude employee [12]. Turner, et al.'s study was funded by the Washington State Department of Labor and Industries, which pays for Washington state workers' compensation insurance [11].
Three studies denied or minimized any potential conflicts of interest [15,16,18]. De Andres specifically noted having independent funding sources with active minimization of the role of manufacturing representatives in the study [15]. Two studies did not disclose whether or not conflicts might have existed [8,17].
One studies did not disclose whether or not conflicts might have existed [17].
Conclusion
FBSS is now one of the most common indications for the utilization of SCS. Evidence for the efficacy of SCS in this indication is accumulating, with most studies demonstrating its efficacy - especially for those patients with leg pain as the predominant symptom. However, a significant weakness in the current available data includes potential bias based on the funding source for most studies. Additionally, it is clear that SCS provides short-term benefit, yet there is no solid evidence that SCS provides any benefit beyond two years of implantation. Another major concern of SCS is its significant placebo effect, which makes the true therapeutic response difficult to judge. Further, it is increasingly important to focus future studies on accurately identifying the patient populations that will best respond to both SCS therapy in general as well as specific stimulation techniques.
Table 1: Characteristics, quality, and main outcomes of included studies.
| Study | Study Design | Study size | Jadad Scale | Summary of Findings |
|---|---|---|---|---|
| North, Jan 2005[8] | RCT | 50 | 3 | SCS provides greater pain relief and patient satisfaction with less analgesic use and loss of function than re-operation for treatment of chronic radicular pain after prior lumbosacral spine surgery |
| North, June 2005[18] | Prospective, controlled | 20 | 0 | Improved pain relief and reduction in analgesic use achieved in both single and double electrode groups |
| North, Nov 2005[17] | RCT | 24 | 1 | Both laminectomy and percutaneous electrode placement achieved significant axial and radial pain relief |
| Kumar, 2007[14] | RCT | 100 | 3 | SCS and CMM is more effective at pain reduction, improved function, and health-related quality of life than CMM alone at 6mo follow-up with greater patient satisfaction |
| Kumar, 2008[10] | RCT | 46 | 3 | SCS and CMM is more effective at pain reduction, improved function, and health-related quality of life than CMM alone at 24mo follow-up with greater patient satisfaction |
| Turner, 2009[11] | Prospective, population-based controlled cohort study | 168 | 0 | No evidence for greater success* of SCS over pain clinic or usual care in workers' compensation patients with FBSS after 6mo. No change in function or analgesic use |
| Schu, 2014[13] | Randomized, double-blind, placebo controlled study | 20 | 5 | Burst stimulation provided significantly greater pain relief over 500 Hz tonic stimulation and placebo stimulation. No statistically significant improvement between groups in function. High patient satisfaction with burst stim |
| Van Havenbergh, 2014[12] | RCT | 15 | 2 | Reduction in axial and radial pain, significance not stated. No significant difference in pain relief between 500 Hz burst stimulation and 1000 Hz burst stimulation |
| van Gorp, 2017[16] | RCT | 52 | 2 | SCS vs. SCS + PNFS: Significant improvement in axial and radial pain for SCS alone. Minimal to moderate patient impression of improvement overall |
| de Andres, 2017[15] | RCT | 60 | 5 | Conventional vs. HFSCS: Statistically significant improvement in axial and radial pain long term and improved quality of life. High patient satisfaction. |
| Al-Kaisy, 2018[9] | Prospective, randomized, sham-controlled, double-blinded, crossover study | 24 | 5 | Sham vs. array of HFSCS: Statistically significant pain relief in all groups, including sham. Moderate patient satisfaction; simsilar between all groups |
RCT = randomized controlled trial; SCS = spinal cord stimulation; CMC = conventional medical management; PNFS = peripheral nerve field stimulation; HFSCS=high frequency spinal cord stimulation; mo= months; *Success was defined as: > 50% pain relief + at least 2-point reduction on RDQ score + reduction in daily opioid medication use
Table 2: Jadad Scale [7].
| Jadad scale for reporting randomized controlled trials | ||
|---|---|---|
| Item | Maximum points | Description |
| Randomization | 2 | 1 point if randomization is mentioned 1 additional point if the method of randomization is appropriate Deduct 1 point if the method of randomization is inappropriate (minimum 0) |
| Blinding | 2 | 1 point if blinding is mentioned 1 additional point if the method of blinding is appropriate Deduct 1 point if the method of blinding is inappropriate (minimum 0) |
| An account of all patients | 1 | The fate of all patients in the trial is known. If there are no data the reason is stated |
References
- Devinsky O. Electrical and magnetic stimulation of the central nervous system. Historical overview. Adv Neurol. 1993; 63:1-16.
- Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: Preliminary clinical report. Anesth Analg. 1967; 46:489-491.
- Melzack R, Wall PD. Pain mechanisms: A new theory. Pain Forum. 1996; 5:3-11.
- Kinfe TM, Pintea B, Vatter H. Is spinal cord stimulation useful and safe for the treatment of chronic pain of ischemic origin? A review. Clin J Pain. 2016; 32:7-13.
- Skolasky RL, Wegener ST, Maggard AM, Riley LH. The impact of reduction of pain after lumbar spine surgery: The relationship between changes in pain and physical function and disability. Spine (Phila Pa 1976). 2014; 39:1426-1432.
- Chan CW, Peng P. Failed back surgery syndrome. Pain Med. 2011; 12:577-606.
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Controlled Clinical Trials. 1996; 17:1-12.
- North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: A randomized, controlled trial. Neurosurgery. 2005; 56:98-107.
- Al‐Kaisy A, Palmisani S, Pang D, et al. Prospective, randomized, Sham‐Control, double blind, crossover trial of subthreshold spinal cord stimulation at various kilohertz frequencies in subjects suffering from failed back surgery syndrome (SCS frequency study). Neuromodulation: Technology at the Neural Interface. 2018; 21:457-465.
- Kumar K, Taylor RS, Jacques L, et al. The effects of spinal cord stimulation in neuropathic pain are sustained: A 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery. 2008; 63:762-770.
- Turner JA, Hollingworth W, Comstock BA, Deyo RA. Spinal cord stimulation for failed back surgery syndrome: Outcomes in a workers' compensation setting. Pain. 2010; 148:14-25.
- Van Havenbergh T, Vancamp T, Van Looy P, Vanneste S, De Ridder D. Spinal cord stimulation for the treatment of chronic back pain patients: 500‐Hz vs. 1000‐Hz burst stimulation. Neuromodulation: Technology at the Neural Interface. 2015; 18:9-12.
- Schu S, Slotty PJ, Bara G, Knop M, Edgar D, Vesper J. A prospective, randomised, double‐blind, placebo‐controlled study to examine the effectiveness of burst spinal cord stimulation patterns for the treatment of failed back surgery syndrome. Neuromodulation: Technology at the Neural Interface. 2014; 17:443-450.
- Kumar K, Taylor RS, Jacques L, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: A multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007; 132:179-188.
- De Andres J, Monsalve-Dolz V, Fabregat-Cid G, et al. Prospective, randomized blind effect-on-outcome study of conventional vs. high-frequency spinal cord stimulation in patients with pain and disability due to failed back surgery syndrome. Pain medicine (Malden, Mass.). 2017; 18:2401-2421.
- van Gorp, Eric-Jan J. A. A, Teernstra O, Aukes HJ, et al. Long-term effect of peripheral nerve field stimulation as add-on therapy to spinal cord stimulation to treat low back pain in failed back surgery syndrome patients: A 12-month follow-up of a randomized controlled study. Neuromodulation: Technology at the Neural Interface. 2018 .
- North RB, Kidd DH, Petrucci L, Dorsi MJ. Spinal cord stimulation electrode design: A prospective, randomized, controlled trial comparing percutaneous with laminectomy electrodes: Part II-clinical outcomes. Neurosurgery. 2005; 57:990-996.
- North RB, Kidd DH, Olin J, Sieracki JN, Petrucci L. Spinal cord stimulation for axial low back pain: A prospective controlled trial comparing 16-contact insulated electrodes with 4-contact percutaneous electrodes. Neuromodulation : journal of the International Neuromodulation Society. 2006; 9:56.
- Simon, Jeremy, MD|McAuliffe, Matthew, MD|Shamim, Fehreen, MD|Vuong, Nancy, MD|Tahaei, Amir, MD. Discogenic low back pain. Physical Medicine and Rehabilitation Clinics of North America. 2014; 25:305-317.
- Harris I, Mulford J, Solomon M, van Gelder JM, Young J. Association between compensation status and outcome after surgery: A meta-analysis. JAMA. 2005; 293:1644-1652.
- Gross DP, Battie MC. Work-related recovery expectations and the prognosis of chronic low back pain within a workers' compensation setting. J Occup Environ Med. 2005; 47:428-433.
- Moens M, Goudman L, Brouns R, et al. Return to work of patients treated with spinal cord stimulation for chronic pain: A systematic review and meta-analysis. Neuromodulation. 2018; .
- Celestin J, Edwards RR, Jamison RN. Pretreatment psychosocial variables as predictors of outcomes following lumbar surgery and spinal cord stimulation: A systematic review and literature synthesis. Pain medicine (Malden, Mass.). 2009; 10:639-653.
- Fama CA, Chen N, Prusik J, et al. The use of preoperative psychological evaluations to predict spinal cord stimulation success: Our experience and a review of the literature. Neuromodulation: Technology at the Neural Interface. 2016; 19:429-436.
- Fishbain DA, Lewis JE, Cutler R, Cole B, Steele Rosomoff R, Rosomoff HL. Does smoking status affect multidisciplinary pain facility treatment outcome? Pain Medicine. 2008; 9:1081-1090.
- Hooten WM, Townsend CO, Bruce BK, et al. Effects of smoking status on immediate treatment outcomes of multidisciplinary pain rehabilitation. Pain medicine (Malden, Mass.). 2009; 10:347-355.
- Fillingim, Roger B.|King, Christopher D.|Ribeiro-Dasilva, Margarete C.|Rahim-Williams, Bridgett|Riley, Joseph L. Sex, gender, and pain: A review of recent clinical and experimental findings. Journal of Pain. 2009; 10:447-485.
- Niesters M, Dahan A, Kest B, et al. Do sex differences exist in opioid analgesia? A systematic review and meta-analysis of human experimental and clinical studies. Pain. 2010; 151:61-68.
- Keogh E, McCracken LM, Eccleston C. Do men and women differ in their response to interdisciplinary chronic pain management? Pain. 2005; 114:37-46.
- David R. Williams, Risa Lavizzo-Mourey, Rueben C. Warren. The concept of race and health status in america. Public Health Reports (1974-). 1994; 109:26-41.
- Fisher TL, Burnet DL, Huang ES, Chin MH, Cagney KA. Cultural leverage. Medical Care Research and Review. 2007; 64:282S.
- Chin MH, Walters AE, Cook SC, Huang ES. Interventions to reduce racial and ethnic disparities in health care. Med Care Res Rev. 2007; 64:28S.
- Witzig R. The medicalization of race: Scientific legitimization of a flawed social construct. Annals of Internal Medicine. 1996; 125:675.
- Anderson, Karen O.|Green, Carmen R.|Payne, Richard. Racial and ethnic disparities in pain: Causes and consequences of Unequal care. Journal of Pain. 2009; 10:1187-1204.
- Cintron A, Morrison RS. Pain and ethnicity in the united states: A systematic review. Journal of palliative medicine. 2006; 9:1454-1473.
- Green CR, Anderson KO, Baker TA, et al. The unequal burden of pain: Confronting racial and ethnic disparities in pain. Pain Medicine. 2003; 4:277-294.
- Edwards CL, Fillingim RB, Keefe F. Race, Ethnicity and Pain. Pain. 2001; 94:133-137.
- Phelan JC, Link BG. Is racism a fundamental cause of inequalities in health? Annual Review of Sociology. 2015; 41:311-330.
- Gee GC, Ford CL. Structural racism and health inequities. Du Bois Review: Social Science Research on Race. 2011; 8:115-132.
- Reyes-Gibby CC, Aday LA, Todd KH, Cleeland CS, Anderson KO. Pain in aging community-dwelling adults in the united states: Non-hispanic whites, non-hispanic blacks, and hispanics. J Pain. 2007; 8:75-84.
- Jones CP. Levels of racism: A theoretic framework and a gardener's tale. American Journal of Public Health. 2000; 90:1212-1215.
Table of Contents
- Abstract
- Introduction
- Methods
- Results
- Summary of Efficacy
- Analgesic use
- Functional change
- Quality of life
- Patient satisfaction
- Patient selection consideration overview
- Working Status
- Psychological Health
- Smoking Status
- Sex
- Race
- Discussion
- Working Status
- Psychological Health
- Smoking Status
- Sex
- Race
- Limitations
- Study Bias
- Conclusion
- Figure 1
- Table 1
- Table 2
- References