Translational Perioperative and Pain Medicine (ISSN: 2330-4871)
ARTICLE DOI: 10.31480/2330-4871/013
REVIEW ARTICLE OPEN ACCESS
Supine Hypotensive Syndrome: A Comprehensive Review of Literature
Pei-Shan Zhao , MD, PhD
Department of Anesthesiology, Tufts Medical Center, Tufts University School of Medicine
Pei-Shan Zhao, MD, PhD, Department of Anesthesiology, Tufts Medical Center, Tufts University School of Medicine, Boston, MA 02111, Email: pzhao@tuftsmedicalcenter.org
Received: September 01, 2014 | Accepted: October 10, 2014 | Published: October 20, 2014
Citation: Pei-Shan Zhao. Supine Hypotensive Syndrome: A Comprehensive Review of Literature. Transl Perioper & Pain Med 2014, 1(2): 22-26.
Abstract
While "supine hypotensive syndrome" is well known to medical professionals in the United States, this may not be recognized well in other countries. In the "No Pain Labor & Delivery-Global Health Initiative" trips, it was observed that the parturient lying on their backs during laboring, resulting in hypotensive episodes and non-reassuring fetal heart rates resulted in subsequent misjudgment of the need for cesarean delivery by clinicians. As part of the educational effort, literature were searched between 1950 and 2014 with key word "supine hypotensive syndrome", "supine hypotension syndrome", "late pregnancy" in PubMed to review the history, clinical presentations and management of supine hypotensive syndrome. The emphasis is that supine hypotensive syndrome occurs more than expected, and that left uterine displacement is not optional after 20 or more weeks of pregnancy.
History
In September, 1953, a full-term young multigravida came to Emergency Room (ER) at Parkland hospital, University of Texas with acute right lower quadrant pain. She became pale and sweated profoundly whenlying supine on the examining table. Her blood pressure (BP) was unmeasurable and her pulse was 160 beats per minute (bpm). Her BP was 120/70 mmHg on arrival at the hospital. It was felt that the patient had a ruptured uterus and immediate laparotomy was performed. A normal intrauterine term pregnancy was found and a living baby was born. What caused this woman's preoperative shock? A few days later, another pregnant woman presented to ER with BP 65/40 mmHg when lying flat. But, unlike the previous patient, she insisted on turning to her side. Her BP rose to 132/80 mmHg after her turning. She was asked to return to supine position. A few minutes later, her BP dropped to 60/35 mmHg. Again she refused to remain supine and insisted on turning to her side. These are the 2 cases Dr. Howard et al. presented first in their 1953 paper [1].
Actually, in the early 1930s, Gideon Ahltorp, a Sweden obstetrician, reported that women suffered symptoms of shock when they lay flat in late pregnancy [2]. It has been noticed that pregnant women have elevated venous pressure in their lower extremities [3] and lowered arterial pressure when they are on supine position [4]. Ahltorp concluded that the "cardiac insufficiency" only occurred when the gravid uterus lay on the posterior wall of the abdomen and suggested that either pathologic elevation of the diaphragm, occlusion of the inferior vena cava (IVC), or a "utero-cardiac neurologic reflex" causing the shock syndrome. Later, many case of "supine hypotension during pregnancy" were reported [5, 6]. However, Howard's group was the first to coin the term "supine hypotensive syndrome" and published their study on dogs and humans in English language literature [1]. They found that 11.2% (18/160) of pregnant women at term had this "supine hypotensive syndrome" (systolic BP decreased 30% of baseline or observed value was 80 mmHg or less) and the shock symptoms occurred 3-7 minutes after the patients lay on their backs. Since compression on the IVC must cause elevation of femoral venous pressure, Howard et al. measured femoral venous pressure of term pregnant women and showed that the femoral venous pressure elevated significantly (average 24 cm H2O) only while the women lay supine.
The femoral venous pressures were back to normal when women turned to either side.
After the abdomen was opened at cesarean delivery (CD) with women lying supine, the femoral venous pressures were still 24 cm H2O and fell to normal limits (average 11 cm H2O) by simply lifting the pregnant uterus off the IVC. Ten years later, Scott and Kerr [7] measured IVC pressures in a patient undergoing CD and again confirmed that when a pregnant woman lay supine, the IVC pressure was always high, ranging from 20 to 25 cm H2O (normal non-pregnant values were 4 to 8 cm H2O). Respiratory pressure changes were not transmitted from the thorax to the distal part of the IVC. Even when the woman took deep breaths there were virtually no detectable pressure changes. The IVC pressure was considerably reduced when the patient turned to her side or when the uterus was lifted forward manually at CD before delivering the baby.
The relationship between IVC occlusion and venous return to the right atrium was also studied on patients undergoing elective CD. When the patient lay supine the IVC pressure varied from 20 to 25 cm H2O and the right atrial pressure ranged from 3 to 5 cm H2O. After the fetuses were delivered the IVC pressure fell to non-pregnant levels and the right atrial pressure rose. In another words, compression of the IVC leads to decreased venous return and reduced right atrial diastolic pressure, resulting in a 25% decrease in cardiac output [7].
Consistent with the previous hemodynamic studies, Kerr et al. 8 performed radiologic studies of the IVC flow on patients (32-40 weeks of pregnancy) who underwent elective CD. After injection of 20 ml of 60% "Urografin" into bilateral femoral veins, they found complete obstructions at the level of the IVC bifurcation in 10 of 12 patients. The compression was not a single point near the origin of the IVC, but evenly distributed throughout the length of the IVC as high as the fundus of the uterus. A partial obstruction was noticed in the other two cases with the left common iliac being more markedly affected than the right. The venous return passed by means of the ascending lumbar veins and the complex of veins surrounding the spinal canal. The previous obstructed IVC in all patients appeared normally patent on the subsequent films after their CDs.
In the paper "supine hypotensive syndrome in late pregnancy" published in 1953, Howard did not think aorta was compressed by the gravid uterus because of its higher pressure and thicker wall compared to that of the IVC. However, 15 years after researches on IVC compression, in 1968, Bieniarz et al.9 showed that partial compression of the aorta occurred when women lay supine in late pregnancy or during labor. After injection of a contrast (Hypaque), serial abdominopelvic arteriograms demonstrated that the sub-renal part of the aorta was displaced laterally most often to the left-and was less densely opacified at the region of the lumbar lordosis (L4-5). Therefore, not only the IVC, but also lower part of the aorta is compressed when women lie supine during late pregnancy. Later, a study on arterial pressure measurement indicated that 60% women in labor had significant reduction in femoral arterial pressure while 18% had reduction in brachial arterial pressure when lying on their backs. That is, the presence of normal brachial arterial pressure in the mother does not rule out aortic compression by the gravid uterus with the risk of utero-placental hypo-perfusion and fetal impairmen [10].
In 1974, based on the previous studies, Marx GF, a professor of Anesthesiology at Albert Einstein College of Medicine, New York, first proposed the concept of "aorto-caval compression" to explain the "supine hypotensive syndrome" [11].
Clinical manifestations
Incidence
In their landmark study, Howard et al. [1] found that 11.2% (18/160) of full-term pregnant women had "supine hypotensive syndrome". Holmes [12] examined 500 pregnant women of 36 or more weeks (180 parous and 320 primigravida) during their routine prenatal visits, however, and found that 31.6 % of pregnant women experienced more than 20% reduction in systolic BP, including 3.6% of patients with systolic BP decreased more than 40%. Neuraxial anesthesia/analgesia cause sympathetic blockade. Theoretically, the incidence of "supine hypotensive syndrome" would be higher in parturients with neuraxial analgesia, but there has been no such report in the literature.
Symptoms and signs
Although it has been reported that the "supine hypotensive syndrome" occurred within 30 seconds or after 30 minutes supine, the BP usually decreases 3-10 minutes after the patients lie on their backs. The main reason is that the pooling of blood in the lower extremities, resulting in decreased venous return and reduction in cardiac output all takes time. Patient complaints include: nausea, vomiting, abdominal/chest discomfort or pain, numbness or paresthesia of the limbs, visual disturbances, tinnitus, headache, dizziness, restlessness, and syncope. Patients like to hold their knees up. Taking a deep breath may partially alleviate hypotension [1, 2, 12]. Signs consist of pale or cyanosis, clammy skin, sweating, muscle twitching, yawning, hyperpnea or dyspnea, low BP, tachycardia or bradycardia etc. In severe cases, there may be incontinence, convulsions and loss of consciousness [2].
In the study of the incidence of "supine hypotensive syndrome", Holmes [12] noticed that some patients, particular those with more severe hypotension (systolic BP decreased > 40%), experienced bradycardia instead of tachycardia, indicating severe reduction of venous return. Bradycardia is caused by atrial reflex, also called Bainbridge Reflex named after Francis Arthur Bainbridge, a British physiologist. In 1915, Bainbridge discovered atrial reflex in the experiments on dogs which showed an increase in blood flow to the heart produced tachycardia. In 1982, researchers confirmed the existence of the Bainbridge reflex in humans [13].
Briefly, atrial reflex is a positive feedback mechanism. As the pressures in the superior and inferior vena cava increase as a result of increased venous return, the atrial pressure raised. This stimulates the stretch receptors in the atrium and (vena cava and pulmonary) venous system. Activation of these stretch receptors lead to increased sympathetic activity and decreased parasympathetic tone via the vagus nerve to the sinoatrial node. In order to protect the venous system from too much pressure, the heart pumps blood to arteries as soon as possible by compensatory tachycardia. In his original study, Bainbridge did not describe the bradycardia response to decreased venous return. However, this was confirmed later by others and named as "reverse Bainbridge reflex". Recently, it was suggested, a full Bainbridge reflex (increase in heart rate (HR) in response to increased venous return and decrease in HR in response to decreased venous return), also known as "cardiopulmonary reflex [14].
The second cardiovascular reflex, contrast to atrial reflex, arterial "baroreceptor reflex", was described about 8 or 9 years after Bainbridge's study. In humans, baroreceptor reflex is more dominant than Bainbridge reflex because of the need to compensate for the changes in BP that accompany changes in posture. The effect of Bainbridge reflex on HR is in opposition to that of "baroreceptor reflex". Bainbridge reflex is more dominant when venous pressure (not arterial pressure) increases due to the increased venous return, leading to tachycardia (positive feedback); Baroreceptor reflex is more dominant when arterial pressure (not venous pressure) decreases, leading to tachycardia (negative feedback).
The third cardiovascular reflex is "Bezold–Jarisch reflex" which is a triad of bradycardia, hypotension, and apnea/hypopnea after IV injection of veratrum alkaloids in animal experiments. The receptors of "Bezold–Jarisch reflex" are located in the ventricular walls, not the great vessels. It has been reported that, during mild to moderate hemorrhage, the baroreceptor reflex is activated to increase the HR and myocardial contractility, and peripheral vascular resistance in attempt to normalize BP. During severe hemorrhage (approximately > 30% of blood volume), the nearly empty ventricles contract vigorously. This mechanical stimulation activates ventricular receptors (not the stretch receptors in the atrium and the vena cava); leading to reflex bradycardia, decrease in total peripheral resistance and arterial blood pressure [14].
Studies have indicated that the arterial baroreceptor reflex is impaired by volatile and IV anesthetics, but little information is available on the effect of these drugs on the Bainbridge reflexes. If the anesthetic drugs have less depressive effect on the Bainbridge reflexes than on the arterial baroreceptor reflex, it is plausible that the Bainbridge reflex would be more dominant in the anesthetized patient than in the conscious patient. Considering the complicated interactions among the various cardiovascular reflexes, it is difficult to clearly separate the contribution of each reflex to a specific clinical scenario. Based on clinical observations and current literature, most experts believe that the decreases in HR observed during severe hemorrhage appear to be mediated by the Bezold–Jarisch reflex while decreases in HR during spinal anesthesia, epidural anesthesia, and controlled hypotension seems to be mediated by reverse Bainbridge reflex [14].
Implications and evidences of (reverse) Bainbridge reflex for bradycardia during neuraxial anesthesia/analgesia:
Although the arterial baroreceptor reflex could increase HR when hypotension occurs during neuraxial anesthesia/analgesia, it has been observed that HR either has on change or decreases during high (T4 and above) or low (below T4) spinal anesthesia. The decrease in HR during high spinal anesthesia has been attributed solely to inhibition of the cardiac sympathetic nerves (T1-T4). However, this mechanism cannot explain the bradycardia during low spinal anesthesia, because the sympathetic innervation to the heart is intact.
In a patient who has hypotension and bradycardia during high spinal anesthesia, raising the legs or placing the patient in the head-down position (leading to increase in venous return) will increase both arterial blood pressure and HR. If decrease in HR were only due to the cardiac sympathetic block, such postural changes would not increase the HR.
If the venous return is maintained, the decrease in HR is approximately 10% with complete cardiac sympathectomy, while decrease in HR is much more than 10% during spinal anesthesia.
The extent of decrease in HR during spinal anesthesia correlates with the decrease in arterial blood pressure regardless the level of anesthesia. The severity of hypotension directly correlates with the venous return, and thus right atrial pressure. As mentioned earlier, variations in HR due to changes in venous pressure may be attributed to atrial reflex.
Patients who had hypotension during lower thoracic (T8-T12) epidural anesthesia also had low right atrial pressure but no changes in HR. This is actually because the decrease in HR due to decreased venous return (reverse Bainbridge reflex) balanced the tachycardia from baroreceptor reflex [14].
Complications
Fetal hypoxia [7] and low Apgar score [15]. Kerr [7] observed a parturient who presented a slow and irregular fetal heart rate (FHR) during the first stage of labor. The patient's BP was checked and found to be low before processing intrapartum CD. She was turned to her side and FHR became normal. This case certainly suggests that the postural hypotension in a parturient is sufficient to cause utero-placental hypo-perfusion and fetal hypoxia. We encountered the similar cases during "No Pain Labor & Delivery" trips.
Possible placental abruption[16]. It has been suggested that when the IVC is occluded, the resultant increased venous pressure may be transmitted back to the chorio-decidual space and causes placental separation. To test this hypothesis, an experiment was performed on two patients during CD. The uterus was lifted up and the IVC was manually occluded. In both cases, placental separation did occur. However, some experts argued that placental separation may be caused by obstruction to the uterine veins and not to the IVC when the whole uterus was lifted out of the abdomen [7].
Decreased urine output may result from decreased renal perfusion and compression on the ureters [7].
Cerebral hypoperfusion. Doppler velocimetry showed a 37% decrease in the velocity of blood flow of internal carotid artery when women lie supine during late pregnancy (29-41 weeks), indicating decreased blood supply to the brain [17].
Fatal consequences. General or spinal anesthesia results in sympathetic blockade and decreased compensatory vasoconstriction. Blood pressure may drop precipitously when pregnant women lie supine under anesthesia. Maternal deaths have been reported [12,18,19] at CD under spinal anesthesia which were probably due to circulatory failure as a result of supine hypotensive syndrome in addition to sympathetic blockade. Supine hypotensive syndrome may be fatal even without anesthesia. Recently, De-Giorgio et al. [20] reported a 41-year-old obese (Height 173 cm, weight 128 kg, before pregnancy 113 kg) pregnant woman who was admitted at 37 weeks with suspected gestational diabetes and fetal macrosomia. Nine days after discharged home, her husband, noticing his wife had been resting for 3 hours, entered the bedroom and found her supine with a book on her face, unresponsively. She was reported asystolic cardiac arrest when the paramedics came and was pronounced dead on arrival at the emergency room. A forensic autopsy showed marked congestion of the jugular and subclavian veins; The IVC appeared collapsed with the femoral veins were markedly dilated and filled with blood on both sides; The right atrium and ventricle were both drooping, with irregular hollows on their surface (reduced diastolic filling of the right heart); Both left atrium and ventricle appeared normal; There were no observable vascular thrombotic phenomena at the level of the pulmonary artery; The coronary arteries did not show significant atherosclerotic plaques or thrombotic phenomena; The authors determined that the only plausible cause of both maternal and fetal death was supine hypotensive syndrome.
Clinical management
Left Uterine Displacement
How to manage "supine hypotensive syndrome", resulting from "aortocaval compression"? The fact that supine pregnant women instinctively turn themselves to their sides tells us the answer. Clinical studies also showed that a wedge-shaped cushion placed under the right hip to tilt the pelvis 15°- 30° away from the horizontal may alleviate, although not relieve completely, the compression of the aorta and IVC [21] Why 15 degree? In 1970s, when water or air-filled bags, sand cushions, rubber wedges, or operating table tiling were used to displace the uterus,Crawford [22] displaced the uterus with a rubber wedge that was placed under the left hips of women undergoing selective CD. The upper surface of the wedge was approximately 15° to the horizontal. This uterine displacement effectively reduced the complications of IVC compression as demonstrated by higher Apgar score and lower incidence of neonatal acidosis among infants whose mothers were tilted compared with those whose mothers were in supine position. However, "aorto-caval compression" still exists when the pelvis is tilted at 15°. Even at 30° tilt, there is evidence that cardiac output and lower extremity artery pressure increased when either manual left uterus displacement (LUD) was performed in women who were already tilted or reposition them in full lateral position [21, 23] Therefore, some authors suggested that 45° tilt is more effective [13]. However, patients feel insecure if the operating table is tilted that much. Normal volunteers first expressed concern at a mean angle of 9° (range 4° to 14°). Experts recommended 15° of tilt at CD and 30° during labor [21]. If LUD cannot relieve the symptoms of "aorto-caval compression" or abnormal FHR, the patient should be positioned at full left lateral position [24] or right uterine displacement by lifting the left hip [25]. By all means, the angle and direction of uterine displacement should be adjusted to relieve the symptoms of "aorto-caval compression". Recently, zhou [26] suggested that a lumbar wedge is more effective than a pelvic wedge in preventing hypotension following combined spinal epidural anesthesia. This needs to be confirmed with further studiea. In routine practice, we use a big pillow or rolled blanket to lift both hip and waist, which makes patients more comfortable. It should be noticed that most anesthesia providers overestimate the angle and tend to have less LUD than expected [27].
Figure 1: Left uterine displacement using 1-handed technique (28).
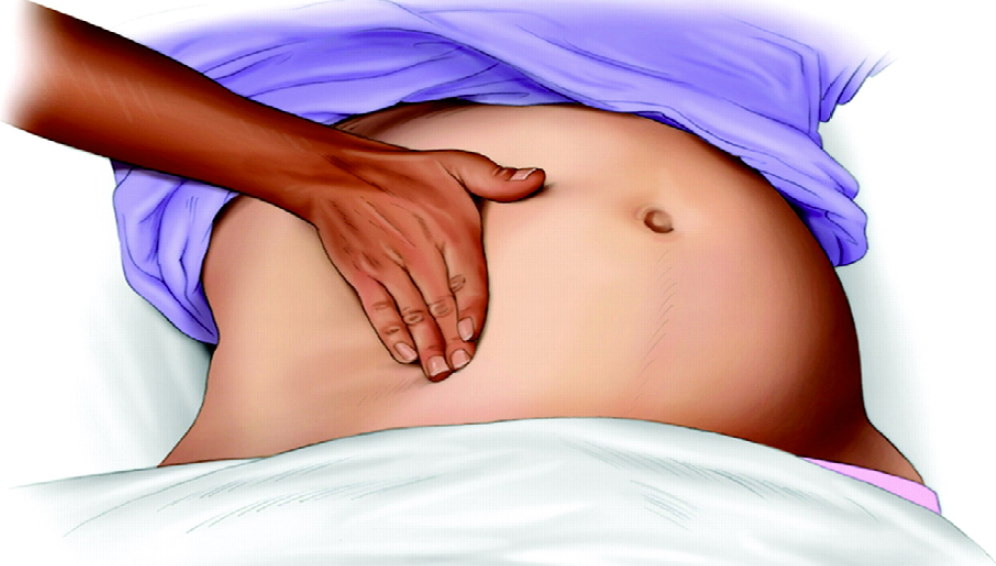
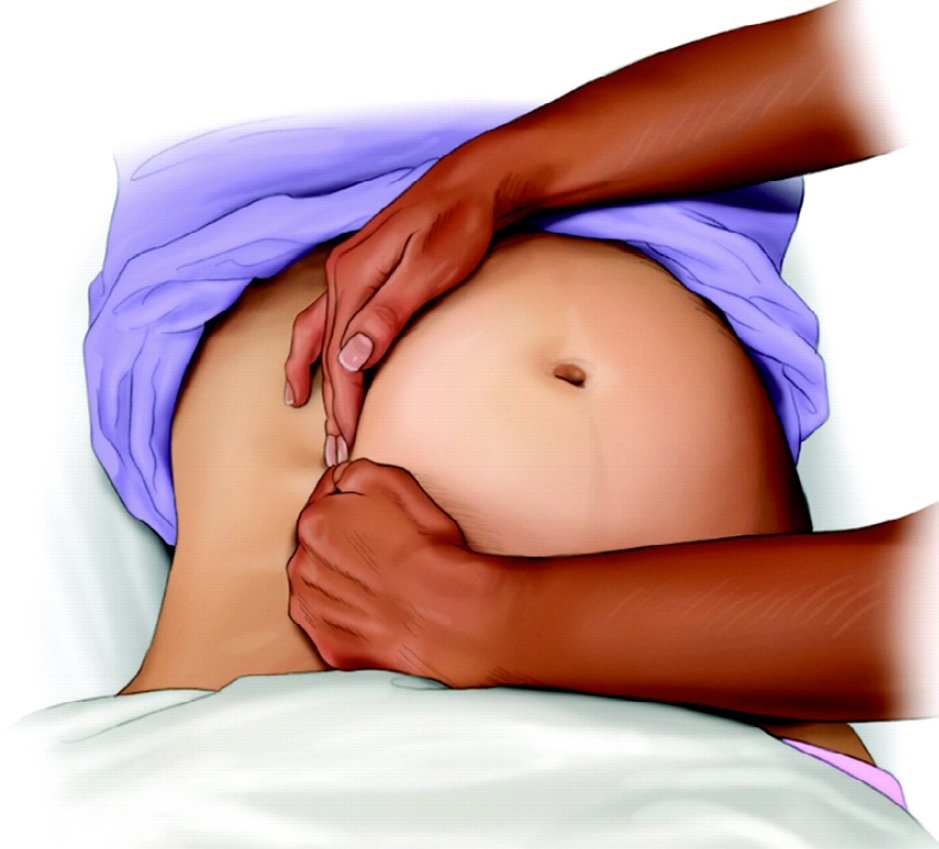
Many studies were undertaken in healthy parturients with uncompromised fetuses. However, the LUD is even more important for fetus at risk, such as pregnant women with hemorrhage, pre-term labor or obesity11. The hemodynamic and cardiovascular effects of uterine compression during cardiac arrest have not been studied, [28] but obviously, prevention of "aorto-caval compression" is critical during cardiopulmonary resuscitation (CPR) for pregnant women. Although chest compressions are feasible in the tilted position, study has shown that the compression force at an angle of 27° is 80% of that in the supine position, [29] which may affect chest compression quality and potentially lower the survival rate. The most effective way to perform aorto-caval decompression is with a manual LUD (Fig. 1, 2 see above) [28] Manual LUD allows the patient to remain supine, which improves airway management, IV access and defibrillation. More importantly, manual LUD with the patient supine enables simultaneous high quality chest compressions, which is essential to maximizing the chance of a successful resuscitation in all patients.
Figure 2: Left uterine displacement with 2-handed technique (28).
When to do and Who needs LUD
Generally speaking, the uterus starts coming out of the pelvis at 12 weeks of pregnancy. By the week 20, most women can feel the gravid uterus at the level of umbilicus. That indicates the uterus enters the abdomen. Women at 20 or more weeks of pregnancy will experience the symptoms of IVC compression when they are supine [2]. It has also showed that, after 20 weeks of pregnancy, renal blood flow decreased and ureters compressed by gravid uterus as shown on renograms, causing decreased urine output [7]. So, pregnant women of 20 or more weeks should never lie supine. When CD is performed or non-obstetric surgery is undertaken after 20 weeks pregnancy, the first thing after the patient lie on the operating table is to put a wedge under the right hip.
Studies have suggested that IVC occlusion in the supine position occurs not in a minority but in the majority of women in late pregnancy. However, supine IVC occlusion does not always cause "supine hypotensive syndrome". The possible explanations are:
Most pregnant women have sufficient arterial and venous collateral circulation to compensate "aorto-caval compression" [7].
Normally, baroreceptor reflex is a rapid negative feedback. It responds to hypotension by increasing the HR and peripheral vascular resistance to maintain normal BP [7].
The shape of the uterus and extent of flaccidity of the uterus are different. The uterus becomes ovoid and firm with each contraction, sitting on the vertebral column. The bonny structure protects the IVC from compressing by the uterus. Between contractions, the flaccid uterus compresses on the IVC with short period of time since normal interval between each contraction is 1-3 minutes while the "supine hypotensive syndrome" occurs 3-10 minutes after the patient lie on the back.
The degree of lumbar lordosis is different. The more lordosis is, the less force the gravid uterus exerts on the IVC [12].
However, we cannot determine whose arterial and venous collateral circulation is efficient and whose baroreceptor reflex is active enough to restore the BP to normal levels. In addition, simultaneous measurement of brachial and femoral arterial blood pressure has shown that femoral hypotension is more frequent than brachia hypotension when women lie supine at term. Femoral hypotension is an indication of lower aorta obstruction. Therefore, normal brachial BP does not necessarily rule out the possibility of utero-placental hypo-perfusion and fetus impairment. For the safety of every mother and baby, it has been recommended that all women [20] or more weeks pregnant should not lie supine with sufficient evidences, which perhaps is the most cost-effective maneuver in the peripartum care cross all specialties.
Disclosure of Funding
None.
References
- Howard KB, Goodson JH, Mengert WF. Supine hypotensive syndrome in late pregnancy. Obstet. Gynec. 1953; 1: 371-7.
- Kinsella SM, Lohmann G. Supine hypotensive syndrome. Obstet Gynecol 1994; 83: 774-88.
- Adolf J, Kriessmann A, Klose BJ, Weidenbach A. The influence of pregnancy on the venous return from the lower extremities (author's transl)]. Med Klin. 1976; 9;71(2): 56-9.
- McRoberts WA Jr. Postural shock in pregnancy. Am J Obstet Gynecol. 1951; 62(3):627-32.
- Brigden W, Howarth S, Sharpey-Schafer EP. Postural changes in the peripheral blood flow of normal subjects with observations on vasovagal fainting reactions as a result of tilting, the lordotic posture, pregnancy and spinal anaesthesia. Clin Sci.1950; 30; 9(2):79-91.
- Scott DB and Kerr MG. Inferior vena caval pressure in late pregnancy. J. Obster. Cynaec. Brit. Cwlth., 1963 70, 1044-9.
- Kerr MG. The mechanical effects of the gravid uterus in late pregnancy. J Obstet Gynaecol Br Commonw. 1965; 72: 513-29.
- Kerr MG, Scott DB, Samuel E. Studies of the Inferior Vena Cava in Late Pregnancy. Brit. med. J.1964; 1: 532-3.
- Bieniarz J, Crottogini JJ, Curuchet E et al. Aorta-Caval compression by the uterus in late human pregnancy. II. An arteriographic study. Am J Obstet Gynecol. 1968; 100: 203-17.
- Eckstein, KL and Marx GF. Aortocaval compression and uterine displacement. Anesthesiology 1974; 40: 92-6.
- Marx GF. Aortocaval compression: incidence and prevention. Bull N Y Acad Med. 1974; 50 (4): 443-6.
- Holmes F. Incidence of the supine hypotensive syndrome in late pregnancy. A clinical study in 500 subjects. J Obstet Gynaecol Br Emp. 1960; 67: 254-8.
- Boettcher DH, Zimpfer M, Vatner SF. Phylogenesis of the Bainbridge reflex. Am J Physiol 1982; 242: R244–6.
- Crystal GJ and Salem MR. The Bainbridge and the "Reverse" Bainbridge Reflexes: History, Physiology, and Clinical Relevance.Anesth Analg 2012;114: 520–32.
- Goodlin RC. Aortocaval compression during cesarean section. A cause of newborn depression. Obstet Gynecol 1971;37(5): 702-5.
- Mengert WF, Goodson JH, Campbell RG, Haynes DM. Observations on the pathogenesis of premature separation of the normally implanted placenta. Am J Obstet Gynecol. 1953;66(5):1104-12.
- Ikeda T, Ohbuchi H, Ikenoue T, Mori N. Maternal cerebral hemodynamics in the supine hypotensive syndrome. Obstet Gynecol. 1992; 79(1): 27-31.
- Holmes F. Spinal analgesia and caesarean section; maternal mortality. J Obstet Gynaecol Br Emp. 1957; 64(2): 229-32.
- Williams B. Collapse from spinal analgesia in pregnancy. Anaesthesia 1958; 13: 448-53.
- De-Giorgio F, Grassi VM, Vetrugno G, d'Aloja E, Pascali VL, Arena V. Supine Hypotensive Syndrome as the Probable Cause of Both Maternal and Fetal Death. J Forensic Sci, November 2012; 57 (6): 1646-9.
- Kinsella SM. Lateral tilt for pregnant women: why 15 degrees? Anaesthesia, 2003; 58: 835–7.
- Crawford JS, Burton M, Davies P. Time and lateral tilt at Caesarean section. Brit. J. Anaesth. 1972; 44: 477-84.
- Paech MJ. Should we take a different angle in managing pregnant women at delivery? Attempting to avoid the 'supine hypotensive syndrome'. Anaesth Intensive Care. 2008; 36(6):775-7.
- Mendonca C, Griffiths J, Ateleanu B, Collis RE. Hypotension following combined spinal epidural anaesthesia for Caesarean section Left lateral position vs. tilted supine position. Anaesthesia 2003; 58: 428–31.
- Hirabayashi Y, Saitoh K, Fukuda H, Shimizu R. An unusual supine hypotensive syndrome during cesarean section: the importance of trying right tilt if there is a poor response to left tilt. Masui. 1994; 43(10):1590-2.
- Zhou ZQ, Shao Q, Zeng Q, Song J, Yang JJ. Lumbar wedge versus pelvic wedge in preventing hypotension following combined spinal epidural anaesthesia for caesarean delivery. Anaesth Intensive Care. 2008; 36(6):835-9.
- Jones SJ, Kinsella SM, Donald FA. Comparison of measured and estimated angles of table tilt at Caesarean section British Journal of Anaesthesia 2003; 90 (1): 86-7.
- Jeejeebhoy FM and Morrison LJ. Maternal Cardiac Arrest: A Practical and Comprehensive Review. Emergency Medicine International 2013; 6: 1-8.
- Rees GAD and Willis BA. "Resuscitation in late pregnancy," Anaesthesia 1988; 43 (5): 347–9.